Abstract
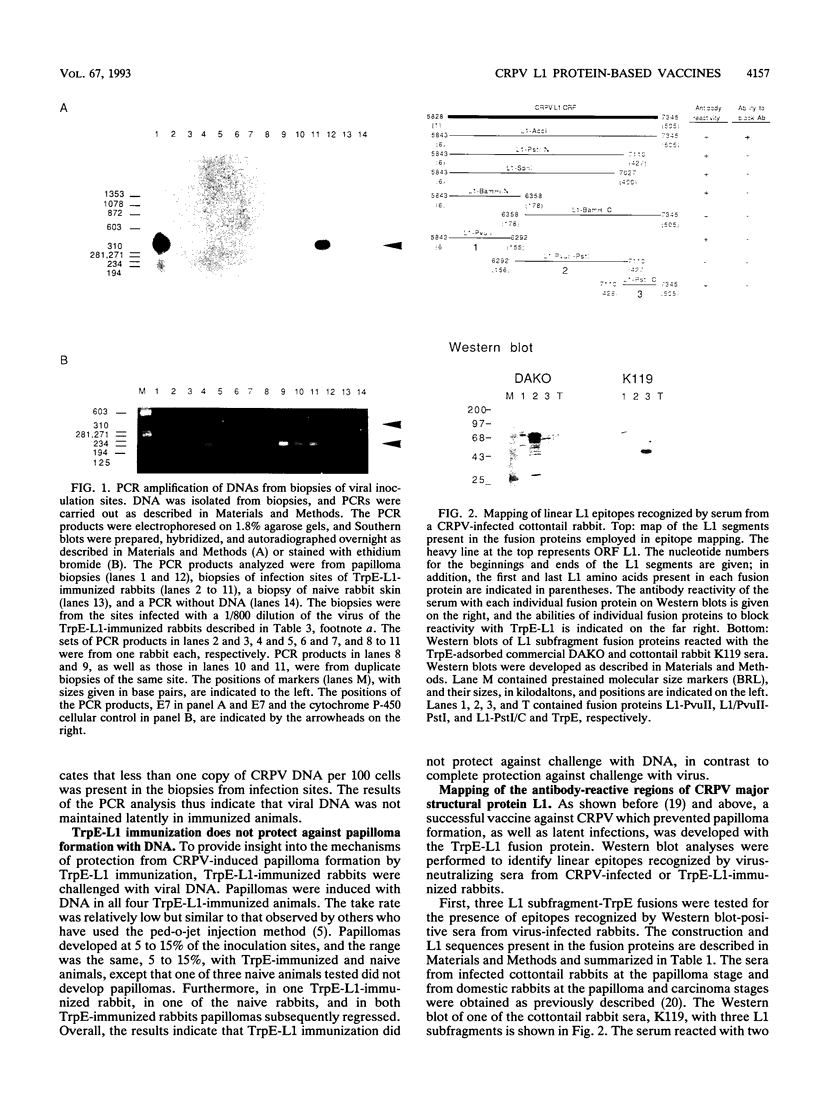
Papillomas induced by the cottontail rabbit papillomavirus (CRPV) progress at a high frequency to carcinomas. In this regard, CRPV and its tumors can serve as an animal model for highly oncogenic human papillomaviruses. We have previously shown that immunization with major structural protein L1 elicits neutralizing antibodies and protects rabbits from papilloma development (Y.-L. Lin, L.A. Borenstein, R. Selvakumar, R. Ahmed, and F.O. Wettstein, Virology 187:612-619, 1992). In this study, we demonstrated that vaccination with the TrpE-L1 fusion protein not only protected rabbits from papilloma development but also prevented latent infection. This was indicated by the failure to amplify CRPV sequences by polymerase chain reaction in biopsies from infection sites of immunized animals. Furthermore, we showed that TrpE-L1 immunization protected rabbits from papilloma formation induced by virus but not from that induced by viral DNA. To explore the possibility of developing vaccines based on L1 subfragments, we mapped the linear L1 epitopes recognized by TrpE-L1-immunized rabbits and by virus-infected rabbits resistant to superinfection. Sera from papilloma-bearing rabbits reacted with one major epitope located at the carboxy-terminal end of L1, between amino acids (aa) 480 and 505. A second epitope, and in some animals a third one, was located in the amino-terminal region, between aa 78 and 101, as well as between aa 37 and 62. Sera from TrpE-L1-immunized animals recognized only one major epitope, located between aa 6 and 37. Immunization of rabbits with L1 subfragment fusion proteins led to seroconversion, but no neutralizing antibodies were produced and the animals were not protected against papilloma formation. The data indicate that a successful papillomavirus vaccine must be based on immunization with full-length native L1 and that further simplification to smaller peptides containing major linear epitopes is not feasible.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandsma J. L., Yang Z. H., Barthold S. W., Johnson E. A. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J Virol. 1990 Jul;64(7):3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Cladel N. M., Patrick S. D., Welsh P. A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990 Nov;64(11):5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Kan N. C., DiAngelo S. L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991 Apr;181(2):572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Defeo-Jones D., Vuocolo G. A., Haskell K. M., Hanobik M. G., Kiefer D. M., McAvoy E. M., Ivey-Hoyle M., Brandsma J. L., Oliff A., Jones R. E. Papillomavirus E7 protein binding to the retinoblastoma protein is not required for viral induction of warts. J Virol. 1993 Feb;67(2):716–725. doi: 10.1128/jvi.67.2.716-725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Marchadier D., Niu H. S., Bunschoten H. M., Otvos L., Jr, Wunner W. H., Ertl H. C., Osterhaus A. D., Koprowski H. Structural and immunological characterization of a linear virus-neutralizing epitope of the rabies virus glycoprotein and its possible use in a synthetic vaccine. J Virol. 1990 Aug;64(8):3804–3809. doi: 10.1128/jvi.64.8.3804-3809.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Dillner L., Utter G., Eklund C., Rotola A., Costa S., DiLuca D. Mapping of linear epitopes of human papillomavirus type 16: the L1 and L2 open reading frames. Int J Cancer. 1990 Mar 15;45(3):529–535. doi: 10.1002/ijc.2910450326. [DOI] [PubMed] [Google Scholar]
- EVANS C. A., GORMAN L. R., ITO Y., WEISER R. S. A vaccination procedure which increases the frequency of regressions of Shope papillomas of rabbits. Nature. 1962 Jan 20;193:288–289. doi: 10.1038/193288a0. [DOI] [PubMed] [Google Scholar]
- EVANS C. A., GORMAN L. R., ITO Y., WEISER R. S. Antitumor immunity in the Shope papilloma-carcinoma complex of rabbits. I. Papilloma regression induced by homologous and autologous tissue vaccines. J Natl Cancer Inst. 1962 Aug;29:277–285. [PubMed] [Google Scholar]
- Evans C. A., Ito Y. Antitumor immunity in the Shope papilloma-carcinoma complex of rabbits. 3. Response to reinfection with viral nucleic acid. J Natl Cancer Inst. 1966 Jun;36(6):1161–1166. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., Smith K. T., O'Neil B. W., Gaukroger J. M., Chandrachud L. M., Grindlay G. J., McGarvie G. M., Campo M. S. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991 Sep;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Jenison S. A., Yu X. P., Valentine J. M., Galloway D. A. Human antibodies react with an epitope of the human papillomavirus type 6b L1 open reading frame which is distinct from the type-common epitope. J Virol. 1989 Feb;63(2):809–818. doi: 10.1128/jvi.63.2.809-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIDER J. W. STUDIES ON THE MECHANISM RESPONSIBLE FOR THE SPONTANEOUS REGRESSION OF THE SHOPE RABBIT PAPILLOMA. Cancer Res. 1963 Oct;23:1593–1599. [PubMed] [Google Scholar]
- Khani S. C., Porter T. D., Fujita V. S., Coon M. J. Organization and differential expression of two highly similar genes in the rabbit alcohol-inducible cytochrome P-450 subfamily. J Biol Chem. 1988 May 25;263(15):7170–7175. [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992 Apr;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Progression from papilloma to carcinoma is accompanied by changes in antibody response to papillomavirus proteins. J Virol. 1993 Jan;67(1):382–389. doi: 10.1128/jvi.67.1.382-389.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Wettstein F. O. The late region differentially regulates the in vitro transformation by cottontail rabbit papillomavirus DNA in different cell types. Virology. 1991 Apr;181(2):637–646. doi: 10.1016/0042-6822(91)90897-k. [DOI] [PubMed] [Google Scholar]
- Orth G., Breitburd F., Favre M. Evidence for antigenic determinants shared by the structural polypeptides of (Shope) rabbit papillomavirus and human papillomavirus type 1. Virology. 1978 Dec;91(2):243–255. doi: 10.1016/0042-6822(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Riggs E. R., Spragion D. E., Muir A. J., Scearce R. M., Randall R. R., McAdams M. W., McKnight A., Clapham P. R., Weiss R. A. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J Virol. 1992 Oct;66(10):5879–5889. doi: 10.1128/jvi.66.10.5879-5889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Glassman D. L., Glassman K. F., Reed D. E., Lum M. A., Marshall R. F., Muscoplat C. C., Faras A. J. Immunization against bovine papillomavirus infection. Ciba Found Symp. 1986;120:136–156. doi: 10.1002/9780470513309.ch10. [DOI] [PubMed] [Google Scholar]
- Sato H., Hirata J., Kuroda N., Shiraki H., Maeda Y., Okochi K. Identification and mapping of neutralizing epitopes of human parvovirus B19 by using human antibodies. J Virol. 1991 Oct;65(10):5485–5490. doi: 10.1128/jvi.65.10.5485-5490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wettstein F. O. Multiple copies of Shope virus DNA are present in cells of benign and malignant non-virus-producing neoplasms. J Virol. 1979 Jun;30(3):891–898. doi: 10.1128/jvi.30.3.891-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa S., Cheung R. C., Pham Q., Robinson W. S., Marion P. L. Peptide mapping of neutralizing and nonneutralizing epitopes of duck hepatitis B virus pre-S polypeptide. Virology. 1991 Mar;181(1):14–21. doi: 10.1016/0042-6822(91)90465-n. [DOI] [PubMed] [Google Scholar]