Abstract
Small DNA viruses have been historically used as probes of cellular control mechanisms of DNA replication, gene expression, and differentiation. Polyomavirus (Py) DNA replication is known to be linked to differentiation of may cells, including myoblasts. In this report, we use this linkage in myoblasts to simultaneously examine (i) cellular differentiation control of Py DNA replication and (ii) an unusual type of cellular and Py DNA synthesis during differentiation. Early proposals that DNA synthesis was involved in the induced differentiation of myoblasts to myotubes were apparently disproved by reliance on inhibitors of DNA synthesis (cytosine arabinoside and aphidicolin), which indicated that mitosis and DNA replication are not necessary for differentiation. Theoretical problems with the accessibility of inactive chromatin to trans-acting factors led us to reexamine possible involvement of DNA replication in myoblast differentiation. We show here that Py undergoes novel aphidicolin-resistant net DNA synthesis under specific conditions early in induced differentiation of myoblasts (following delayed aphidicolin addition). Under similar conditions, we also examined uninfected myoblast DNA synthesis, and we show that soon after differentiation induction, a period of aphidicolin-resistant cellular DNA synthesis can also be observed. This drug-resistant DNA synthesis appears to be subgenomic, not contributing to mitosis, and more representative of polyadenylated than of nonpolyadenylated RNA. These results renew the possibility that DNA synthesis plays a role in myoblast differentiation and suggest that the linkage of Py DNA synthesis to differentiation may involve a qualitative cellular alteration in Py DNA replication.
Full text
PDF





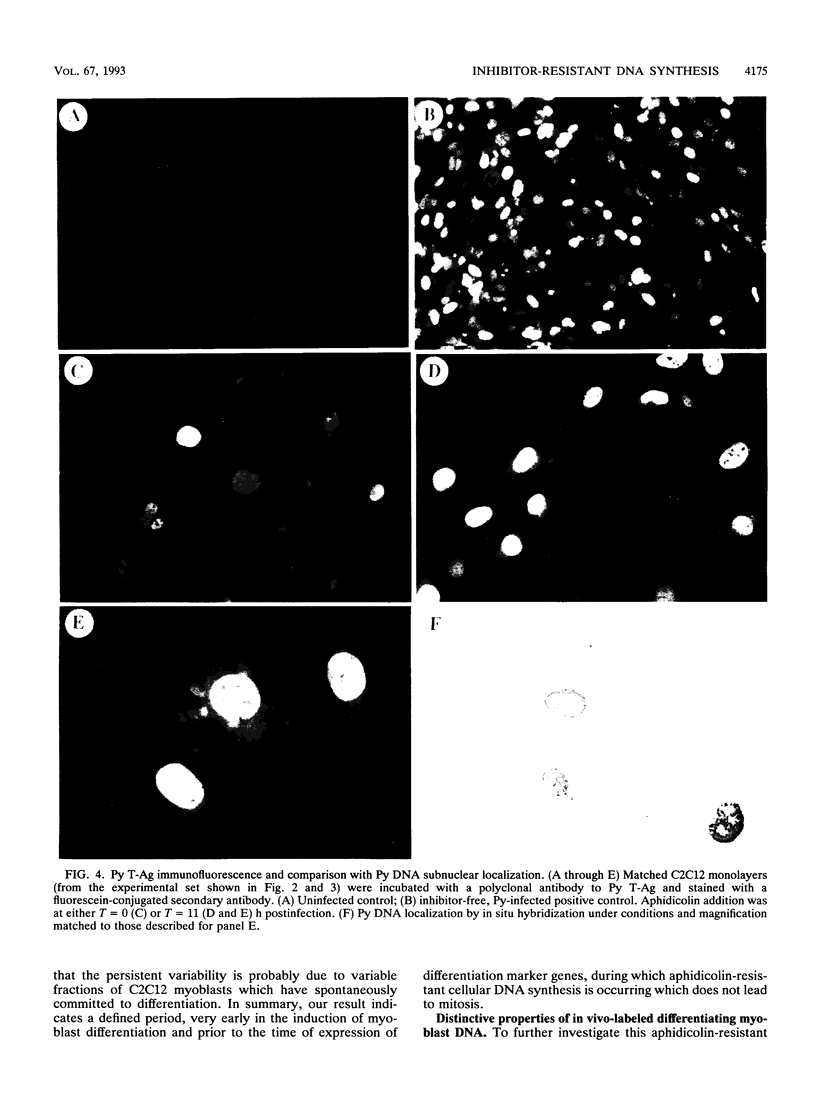
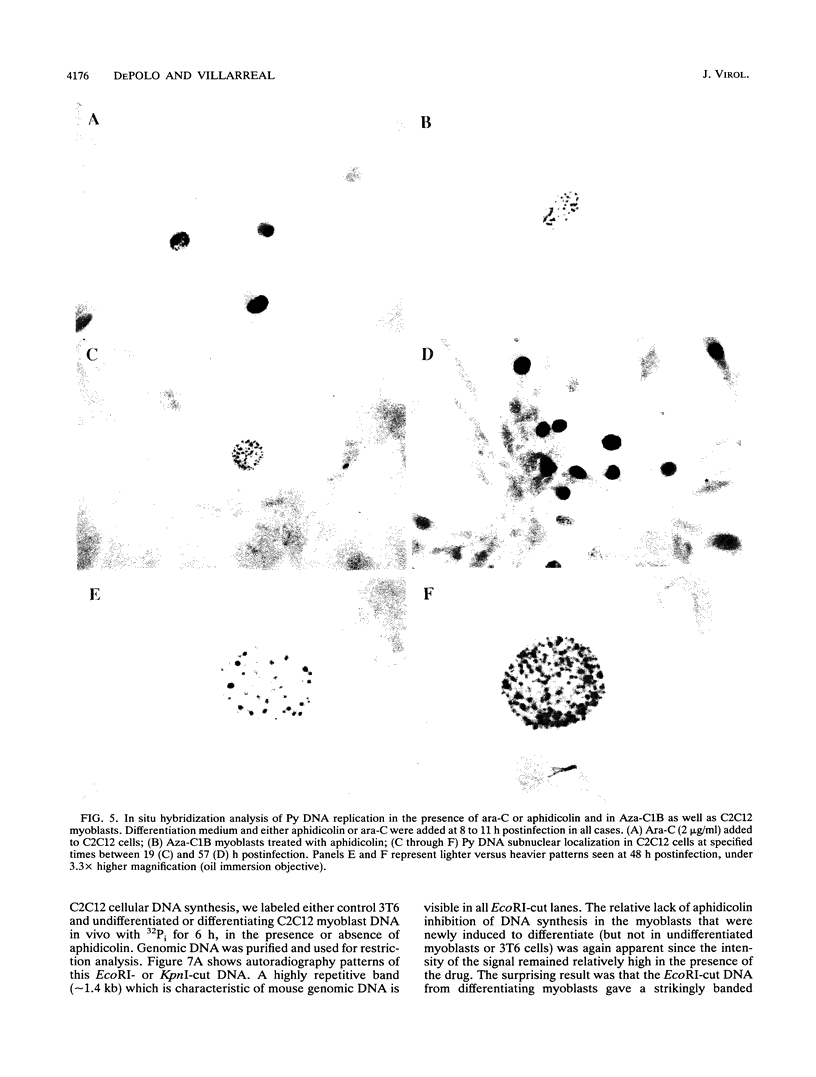
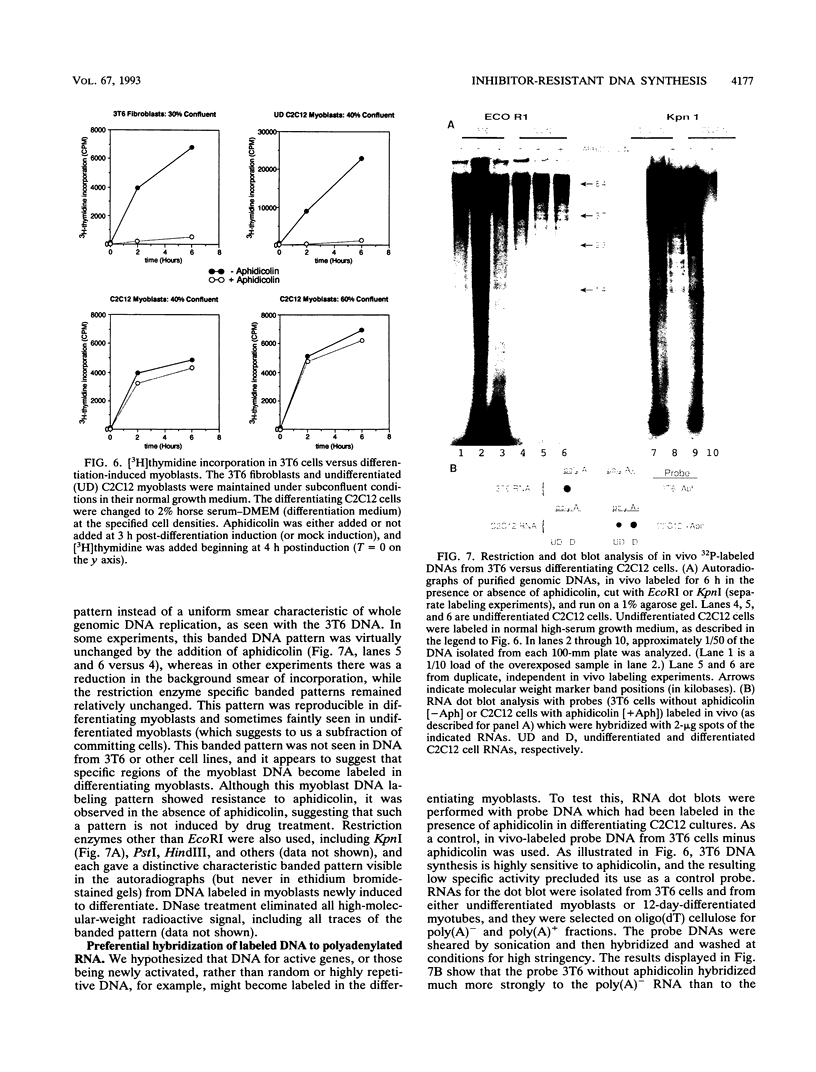




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Gene expression. Chromatin contract to silence. Nature. 1990 Mar 15;344(6263):193–194. doi: 10.1038/344193a0. [DOI] [PubMed] [Google Scholar]
- Amati P. Polyoma regulatory region: a potential probe for mouse cell differentiation. Cell. 1985 Dec;43(3 Pt 2):561–562. doi: 10.1016/0092-8674(85)90225-9. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Cordingley M. G., Wolford R. G., Hager G. L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol Cell Biol. 1991 Feb;11(2):688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio I. A., Shadan F. F., Zhou X. J., Vaziri N. D., Villarreal L. P. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993 Mar;67(3):1424–1432. doi: 10.1128/jvi.67.3.1424-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill C. A., Grochan B. M., Meyn R. E., Bohr V. A., Tofilon P. J. Loss of intragenomic DNA repair heterogeneity with cellular differentiation. J Biol Chem. 1991 Nov 15;266(32):21821–21826. [PubMed] [Google Scholar]
- Blau H. M. How fixed is the differentiated state? Lessons from heterokaryons. Trends Genet. 1989 Aug;5(8):268–272. doi: 10.1016/0168-9525(89)90100-5. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bohr V. A. Gene specific DNA repair. Carcinogenesis. 1991 Nov;12(11):1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- Boyd J. B., Boyd S. F. Influence of bromodeoxyuridine on the stability and function of polytene chromosomes. Chromosoma. 1977 Apr 27;61(1):75–94. doi: 10.1007/BF00292682. [DOI] [PubMed] [Google Scholar]
- Brown D. D. The role of stable complexes that repress and activate eucaryotic genes. Cell. 1984 Jun;37(2):359–365. doi: 10.1016/0092-8674(84)90366-0. [DOI] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Lymphoid and other tissue-specific phenotypes of polyomavirus enhancer recombinants: positive and negative combinational effects on enhancer specificity and activity. Mol Cell Biol. 1986 Jun;6(6):2068–2079. doi: 10.1128/mcb.6.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos R., Villarreal L. P. An SV40 deletion mutant accumulates late transcripts in a paranuclear extract. Virology. 1982 May;119(1):1–11. doi: 10.1016/0042-6822(82)90059-9. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Chiu C. P., Blau H. M. Reprogramming cell differentiation in the absence of DNA synthesis. Cell. 1984 Jul;37(3):879–887. doi: 10.1016/0092-8674(84)90423-9. [DOI] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Darby M. K., Andrews M. T., Brown D. D. Transcription complexes that program Xenopus 5S RNA genes are stable in vivo. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5516–5520. doi: 10.1073/pnas.85.15.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Yamaguchi M., Possenti R., Bradley M. K., DePamphilis M. L. In vitro initiation of DNA replication in simian virus 40 chromosomes. J Biol Chem. 1987 Aug 5;262(22):10863–10872. [PubMed] [Google Scholar]
- Din S., Brill S. J., Fairman M. P., Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990 Jun;4(6):968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- Felsani A., Maione R., Ricci L., Amati P. Coordinate expression of myogenic functions and polyoma virus replication. Cold Spring Harb Symp Quant Biol. 1985;50:753–757. doi: 10.1101/sqb.1985.050.01.093. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J., Bloomer L. S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982 Apr;28(4):781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- Gourdeau H., Fournier R. E. Genetic analysis of mammalian cell differentiation. Annu Rev Cell Biol. 1990;6:69–94. doi: 10.1146/annurev.cb.06.110190.000441. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone function in transcription. Annu Rev Cell Biol. 1990;6:643–678. doi: 10.1146/annurev.cb.06.110190.003235. [DOI] [PubMed] [Google Scholar]
- Han M., Grunstein M. Nucleosome loss activates yeast downstream promoters in vivo. Cell. 1988 Dec 23;55(6):1137–1145. doi: 10.1016/0092-8674(88)90258-9. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Harrington M. A., Gonzales F., Jones P. A. Effect of cellular determination on oncogenic transformation by chemicals and oncogenes. Mol Cell Biol. 1988 Oct;8(10):4322–4327. doi: 10.1128/mcb.8.10.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton K. S., Dhar V., Brown E. H., Iqbal M. A., Stuart S., Didamo V. T., Schildkraut C. L. Replication program of active and inactive multigene families in mammalian cells. Mol Cell Biol. 1988 May;8(5):2149–2158. doi: 10.1128/mcb.8.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L. J., Zhu Y. Y., Schwartz R. J. Cellular localization of muscle and nonmuscle actin mRNAs in chicken primary myogenic cultures: the induction of alpha-skeletal actin mRNA is regulated independently of alpha-cardiac actin gene expression. J Cell Biol. 1988 Jun;106(6):2077–2086. doi: 10.1083/jcb.106.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Role of replication time in the control of tissue-specific gene expression. Am J Hum Genet. 1987 Feb;40(2):151–173. [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Fellini S., Yeoh G., Chi J., Birnbaum J., Okayama M. Lineages, quantal cell cycles, and the generation of cell diversity. Q Rev Biophys. 1975 Nov;8(4):523–557. doi: 10.1017/s0033583500001980. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Weintraub H., Mayne R., Mochan B. The cell cycle, cell lineages, and cell differentiation. Curr Top Dev Biol. 1972;7:229–256. doi: 10.1016/s0070-2153(08)60073-3. [DOI] [PubMed] [Google Scholar]
- Jackson D. A. The organization of replication centres in higher eukaryotes. Bioessays. 1990 Feb;12(2):87–89. doi: 10.1002/bies.950120207. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kayne P. S., Kim U. J., Han M., Mullen J. R., Yoshizaki F., Grunstein M. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell. 1988 Oct 7;55(1):27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Irresistible force meets immovable object: transcription and the nucleosome. Cell. 1991 Nov 29;67(5):833–836. doi: 10.1016/0092-8674(91)90354-2. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Taneja K., Singer R. H. Temporal resolution and sequential expression of muscle-specific genes revealed by in situ hybridization. Dev Biol. 1989 May;133(1):235–246. doi: 10.1016/0012-1606(89)90314-x. [DOI] [PubMed] [Google Scholar]
- Linn S. How many pols does it take to replicate nuclear DNA? Cell. 1991 Jul 26;66(2):185–187. doi: 10.1016/0092-8674(91)90608-2. [DOI] [PubMed] [Google Scholar]
- Lorch Y., LaPointe J. W., Kornberg R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell. 1987 Apr 24;49(2):203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- Losa R., Brown D. D. A bacteriophage RNA polymerase transcribes in vitro through a nucleosome core without displacing it. Cell. 1987 Aug 28;50(5):801–808. doi: 10.1016/0092-8674(87)90338-2. [DOI] [PubMed] [Google Scholar]
- Maione R., Felsani A., Pozzi L., Caruso M., Amati P. Polyomavirus genome and polyomavirus enhancer-driven gene expression during myogenesis. J Virol. 1989 Nov;63(11):4890–4897. doi: 10.1128/jvi.63.11.4890-4897.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. P., Villarreal L. P. Analysis of cellular DNA synthesis during polyoma virus infection of mice: acute infection fails to induce cellular DNA synthesis. Virology. 1992 Feb;186(2):463–474. doi: 10.1016/0042-6822(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Eki T., Yamada M., Prives C., Hurwitz J. Species-specific in vitro synthesis of DNA containing the polyoma virus origin of replication. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6347–6351. doi: 10.1073/pnas.83.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Spradling A. C. Drosophila chorion gene amplification requires an upstream region regulating s18 transcription. Mol Cell Biol. 1986 Dec;6(12):4624–4633. doi: 10.1128/mcb.6.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. Visualization of Drosophila melanogaster chorion genes undergoing amplification. Mol Cell Biol. 1988 Jul;8(7):2811–2821. doi: 10.1128/mcb.8.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset C., Whalen R. G. Induction of myogenic differentiation in serum-free medium does not require DNA synthesis. Dev Biol. 1985 Apr;108(2):284–289. doi: 10.1016/0012-1606(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Prives C., Murakami Y., Kern F. G., Folk W., Basilico C., Hurwitz J. DNA sequence requirements for replication of polyomavirus DNA in vivo and in vitro. Mol Cell Biol. 1987 Oct;7(10):3694–3704. doi: 10.1128/mcb.7.10.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. A pancreas specificity results from the combination of polyomavirus and Moloney murine leukemia virus enhancer. Proc Natl Acad Sci U S A. 1987 Jan;84(2):449–453. doi: 10.1073/pnas.84.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. Genetic analysis of the enhancer requirements for polyomavirus DNA replication in mice. J Virol. 1990 Feb;64(2):476–485. doi: 10.1128/jvi.64.2.476-485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Moreno J. P., Peake M. L., Villarreal L. P. Enhancer dependence of polyomavirus persistence in mouse kidneys. J Virol. 1992 Jun;66(6):3287–3297. doi: 10.1128/jvi.66.6.3287-3297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin G. T. Replication in polytene chromosomes. Results Probl Cell Differ. 1972;4:59–85. doi: 10.1007/978-3-540-37164-9_3. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Structural topography of simian virus 40 DNA replication. J Virol. 1991 May;65(5):2578–2588. doi: 10.1128/jvi.65.5.2578-2588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Kalf G. F. DNA polymerase beta involvement in DNA endoreduplication in rat giant trophoblast cells. J Biol Chem. 1982 Feb 25;257(4):1785–1790. [PubMed] [Google Scholar]
- Silberstein L., Webster S. G., Travis M., Blau H. M. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986 Sep 26;46(7):1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Smithies O. The control of globin and other eukaryotic genes. J Cell Physiol Suppl. 1982;1:137–143. doi: 10.1002/jcp.1041130421. [DOI] [PubMed] [Google Scholar]
- Svaren J., Chalkley R. The structure and assembly of active chromatin. Trends Genet. 1990 Feb;6(2):52–56. doi: 10.1016/0168-9525(90)90074-g. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. H. Asynchronous duplication of chromosomes in cultured cells of Chinese hamster. J Biophys Biochem Cytol. 1960 Jun;7:455–464. doi: 10.1083/jcb.7.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Lassar A. B., Davis R. L., Weintraub H. 5-bromo-2'-deoxyuridine blocks myogenesis by extinguishing expression of MyoD1. Science. 1989 Aug 4;245(4917):532–536. doi: 10.1126/science.2547249. [DOI] [PubMed] [Google Scholar]
- Tseng R. W., Fujimura F. K. Multiple domains in the polyomavirus B enhancer are required for productive infection of F9 embryonal carcinoma cells. J Virol. 1988 Aug;62(8):2890–2895. doi: 10.1128/jvi.62.8.2890-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng R. W., Williams T., Fujimura F. K. Unique requirement for the PyF441 mutation for polyomavirus infection of F9 embryonal carcinoma cells. J Virol. 1988 Aug;62(8):2896–2902. doi: 10.1128/jvi.62.8.2896-2902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turo K. A., Florini J. R. Hormonal stimulation of myoblast differentiation in the absence of DNA synthesis. Am J Physiol. 1982 Nov;243(5):C278–C284. doi: 10.1152/ajpcell.1982.243.5.C278. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Sawadogo M., Roeder R. G. Stability of transcription complexes on class II genes. Mol Cell Biol. 1989 Jan;9(1):342–344. doi: 10.1128/mcb.9.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P. Relationship of eukaryotic DNA replication to committed gene expression: general theory for gene control. Microbiol Rev. 1991 Sep;55(3):512–542. doi: 10.1128/mr.55.3.512-542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. H., Moen P. T., Jr, Fox E., Bodnar J. W. Interactions of minute virus of mice and adenovirus with host nucleoli. J Virol. 1989 Sep;63(9):3651–3660. doi: 10.1128/jvi.63.9.3651-3660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Collins K. L., Simancek P., Russo A., Wold M. S., Virshup D. M., Kelly T. J. Reconstitution of simian virus 40 DNA replication with purified proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8692–8696. doi: 10.1073/pnas.87.22.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Assembly of an active chromatin structure during replication. Nucleic Acids Res. 1979 Oct 10;7(3):781–792. doi: 10.1093/nar/7.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Developmental regulation of two 5S ribosomal RNA genes. Science. 1988 Sep 23;241(4873):1626–1632. doi: 10.1126/science.241.4873.1626. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. New approaches to chromatin function. New Biol. 1990 Mar;2(3):211–218. [PubMed] [Google Scholar]
- Workman J. L., Abmayr S. M., Cromlish W. A., Roeder R. G. Transcriptional regulation by the immediate early protein of pseudorabies virus during in vitro nucleosome assembly. Cell. 1988 Oct 21;55(2):211–219. doi: 10.1016/0092-8674(88)90044-x. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Roeder R. G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1987 Nov 20;51(4):613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Roeder R. G., Kingston R. E. An upstream transcription factor, USF (MLTF), facilitates the formation of preinitiation complexes during in vitro chromatin assembly. EMBO J. 1990 Apr;9(4):1299–1308. doi: 10.1002/j.1460-2075.1990.tb08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E. Control of differentiation in heterokaryons and hybrids involving differentiation-defective myoblast variants. J Cell Biol. 1984 Feb;98(2):436–443. doi: 10.1083/jcb.98.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]