Abstract
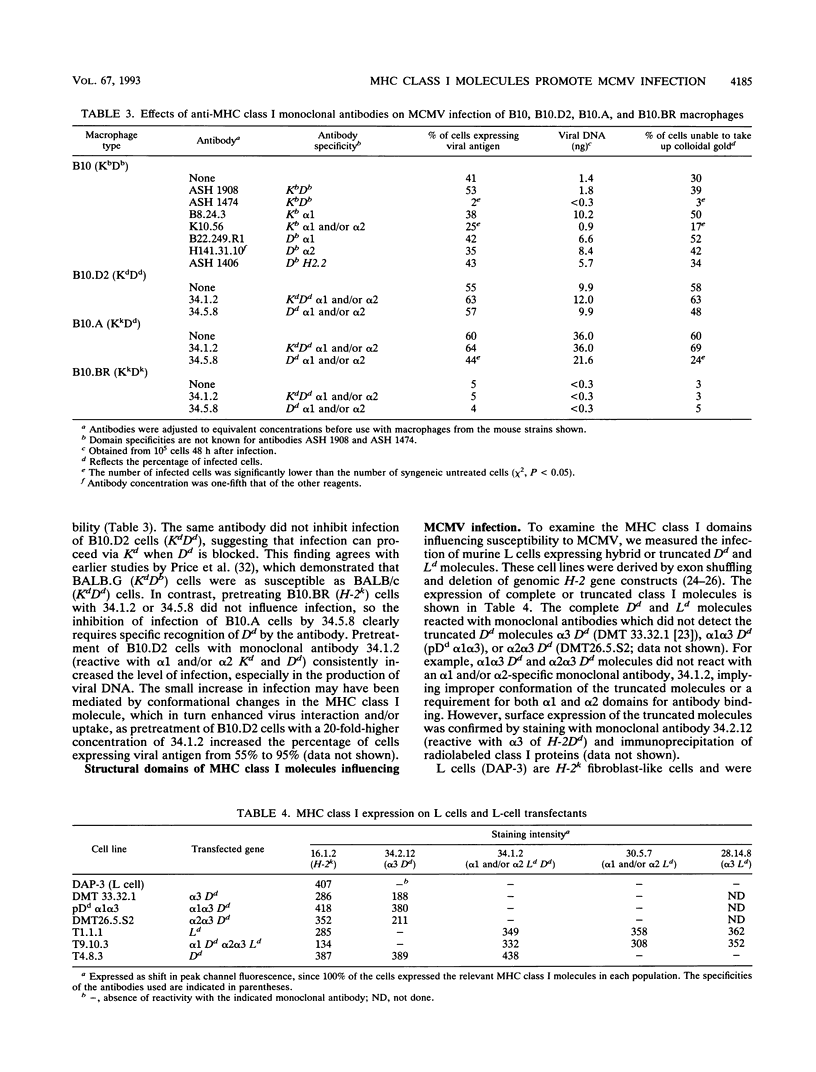
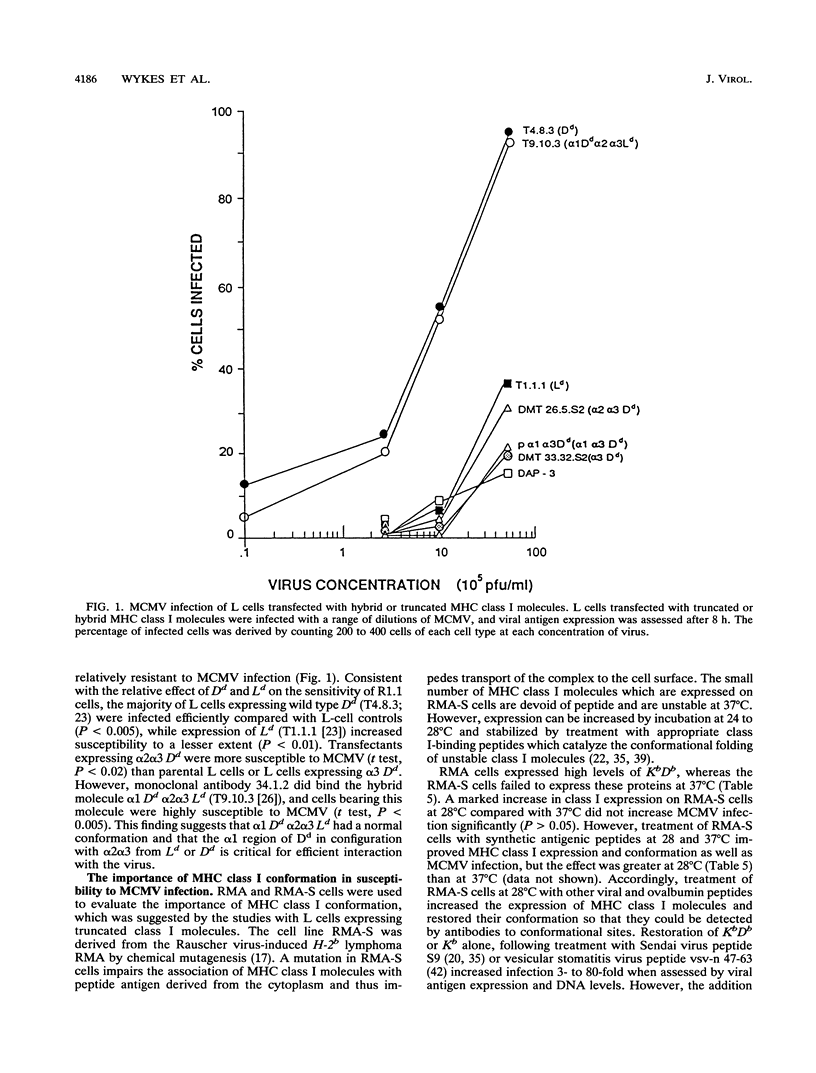
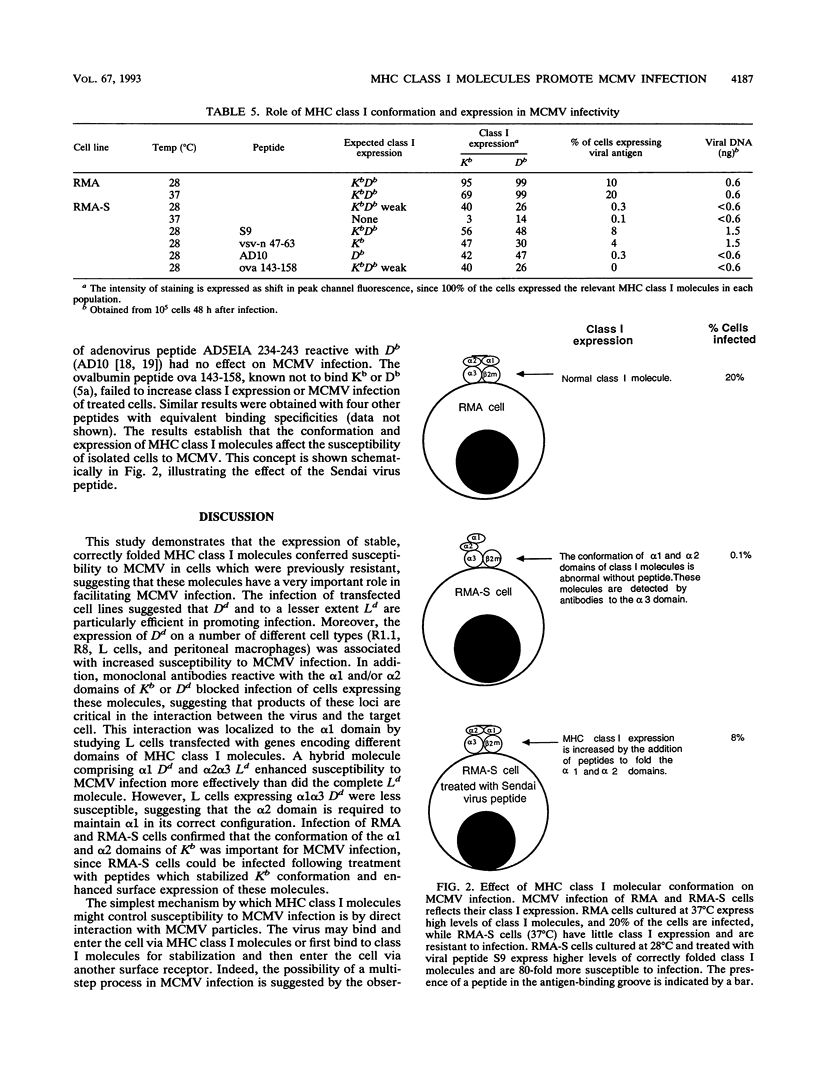
The expression of stable, correctly folded major histocompatibility complex class I molecules conferred susceptibility to murine cytomegalovirus (MCMV) in cells which were previously resistant to infection, demonstrating that these molecules interact critically with MCMV to initiate infection. All class I molecules could potentiate MCMV infection but H-2Dd and Kb molecules were most efficient. Monoclonal antibodies specific for the alpha 1 and/or alpha 2 domains of Dd and Kb inhibited infection. Infection of L cells transfected with hybrid major histocompatibility complex class I molecules demonstrated that allelic control of susceptibility to MCMV mapped to the alpha 1 domain of Dd when in correct configuration with the alpha 2 and alpha 3 domains. In MCMV-resistant RMA-S cells, an improvement in the conformation of class I molecules introduced susceptibility to infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlish J. D., Lahijani R. S., St Jeor S. C. Identification of a putative cell receptor for human cytomegalovirus. Virology. 1990 Jun;176(2):337–345. doi: 10.1016/0042-6822(90)90003-a. [DOI] [PubMed] [Google Scholar]
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Beersma M. F., Wertheim-van Dillen P. M., Geelen J. L., Feltkamp T. E. Expression of HLA class I heavy chains and beta 2-microglobulin does not affect human cytomegalovirus infectivity. J Gen Virol. 1991 Nov;72(Pt 11):2757–2764. doi: 10.1099/0022-1317-72-11-2757. [DOI] [PubMed] [Google Scholar]
- Capps G. G., Van Kampen M., Ward C. L., Zúiga M. C. Endocytosis of the class I major histocompatibility antigen via a phorbol myristate acetate-inducible pathway is a cell-specific phenomenon and requires the cytoplasmic domain. J Cell Biol. 1989 Apr;108(4):1317–1329. doi: 10.1083/jcb.108.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Forrester A., Farrell H., Wilkinson G., Kaye J., Davis-Poynter N., Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992 Jan;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. D., Grundy J. E. Molecular biology and immunology of cytomegalovirus. Biochem J. 1987 Jan 15;241(2):313–324. doi: 10.1042/bj2410313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., Mackenzie J. S., Stanley N. F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981 Apr;32(1):277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Ward P. J., Sanderson A. R., Griffiths P. D. Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):793–803. doi: 10.1099/0022-1317-68-3-793. [DOI] [PubMed] [Google Scholar]
- Harnett G. B., Shellam G. R. Variation in murine cytomegalovirus replication in fibroblasts from different mouse strains in vitro: correlation with in vivo resistance. J Gen Virol. 1982 Sep;62(Pt 1):39–47. doi: 10.1099/0022-1317-62-1-39. [DOI] [PubMed] [Google Scholar]
- Hodgkin P. D., Scalzo A. A., Swaminathan N., Price P., Shellam G. R. Murine cytomegalovirus binds reversibly to mouse embryo fibroblasts: implications for quantitation and explanation of centrifugal enhancement. J Virol Methods. 1988 Dec;22(2-3):215–230. doi: 10.1016/0166-0934(88)90104-8. [DOI] [PubMed] [Google Scholar]
- Hudson J. B. Further studies on the mechanism of centrifugal enhancement of cytomegalovirus infectivity. J Virol Methods. 1988 Feb;19(2):97–108. doi: 10.1016/0166-0934(88)90153-x. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Walker D. G., Altamirano M. Analysis in vitro of two biologically distinct strains of murine cytomegalovirus. Arch Virol. 1988;102(3-4):289–295. doi: 10.1007/BF01310834. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Melief C. J. Fine peptide specificity of cytotoxic T lymphocytes directed against adenovirus-induced tumours and peptide-MHC binding. Int J Cancer Suppl. 1991;6:90–94. doi: 10.1002/ijc.2910470718. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S., Takeshita T., Boehncke W. H., Takahashi H., Boyd L. F., Germain R. N., Berzofsky J. A., Margulies D. H. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991 Jan 3;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Ozato K., Camerini-Otero R. D., Tanaka K., Appella E., Seidman J. G. Expression of H-2Dd and H-2Ld mouse major histocompatibility antigen genes in L cells after DNA-mediated gene transfer. J Immunol. 1983 Jan;130(1):463–470. [PubMed] [Google Scholar]
- Margulies D. H., McCluskey J. Exon shuffling: new genes from old. Surv Immunol Res. 1985;4(2):146–159. doi: 10.1007/BF02918810. [DOI] [PubMed] [Google Scholar]
- McCluskey J., Bluestone J. A., Coligan J. E., Maloy W. L., Margulies D. H. Serologic and T cell recognition of truncated transplantation antigens encoded by in vitro deleted class I major histocompatibility genes. J Immunol. 1986 Feb 15;136(4):1472–1481. [PubMed] [Google Scholar]
- McCluskey J., Boyd L., Foo M., Forman J., Margulies D. H., Bluestone J. A. Analysis of hybrid H-2D and L antigens with reciprocally mismatched aminoterminal domains: functional T cell recognition requires preservation of fine structural determinants. J Immunol. 1986 Dec 15;137(12):3881–3890. [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro D., Paz P., Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992 Jan;186(1):99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Nowlin D. M., Cooper N. R., Compton T. Expression of a human cytomegalovirus receptor correlates with infectibility of cells. J Virol. 1991 Jun;65(6):3114–3121. doi: 10.1128/jvi.65.6.3114-3121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietroboni G. R., Harnett G. B., Bucens M. R. Centrifugal enhancement of human immunodeficiency virus (HIV) and human herpesvirus type 6 (HHV-6) infection in vitro. J Virol Methods. 1989 Apr-May;24(1-2):85–90. doi: 10.1016/0166-0934(89)90010-4. [DOI] [PubMed] [Google Scholar]
- Price P., Gibbons A. E., Shellam G. R. H-2 class I loci determine sensitivity to MCMV in macrophages and fibroblasts. Immunogenetics. 1990;32(1):20–26. doi: 10.1007/BF01787324. [DOI] [PubMed] [Google Scholar]
- Price P., Winter J. G., Nikoletti S., Hudson J. B., Shellam G. R. Functional changes in murine macrophages infected with cytomegalovirus relate to H-2-determined sensitivity to infection. J Virol. 1987 Nov;61(11):3602–3606. doi: 10.1128/jvi.61.11.3602-3606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Smiley M. L., Mar E. C., Huang E. S. Cytomegalovirus infection and viral-induced transformation of human endothelial cells. J Med Virol. 1988 Jun;25(2):213–226. doi: 10.1002/jmv.1890250212. [DOI] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. Human cytomegalovirus binding to fibroblasts is receptor mediated. J Virol. 1989 Sep;63(9):3991–3998. doi: 10.1128/jvi.63.9.3991-3998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. The human cytomegalovirus receptor on fibroblasts is a 30-kilodalton membrane protein. J Virol. 1990 Jun;64(6):2484–2490. doi: 10.1128/jvi.64.6.2484-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Turco M. C., de Felice M., Corbo L., Giarrusso P. C., Yang S. Y., Ferrone S., Venuta S. Enhancing effect of anti-HLA class I monoclonal antibodies on T cell proliferation induced via CD2 molecule. J Immunol. 1988 Oct 1;141(7):2275–2281. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Walker D., Hudson J. Analysis of immediate-early and early proteins of murine cytomegalovirus in permissive and nonpermissive cells. Arch Virol. 1987;92(1-2):103–119. doi: 10.1007/BF01310066. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Barber B. H., Flavell R. A., Allen H. Role of beta 2-microglobulin in the intracellular transport and surface expression of murine class I histocompatibility molecules. J Immunol. 1989 Apr 15;142(8):2796–2806. [PubMed] [Google Scholar]
- Wykes M. N., Price P., Shellam G. R. The effects of beta-2-microglobulin on the infectivity of murine cytomegalovirus. Arch Virol. 1992;123(1-2):59–72. doi: 10.1007/BF01317138. [DOI] [PubMed] [Google Scholar]



