Abstract
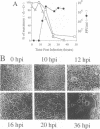
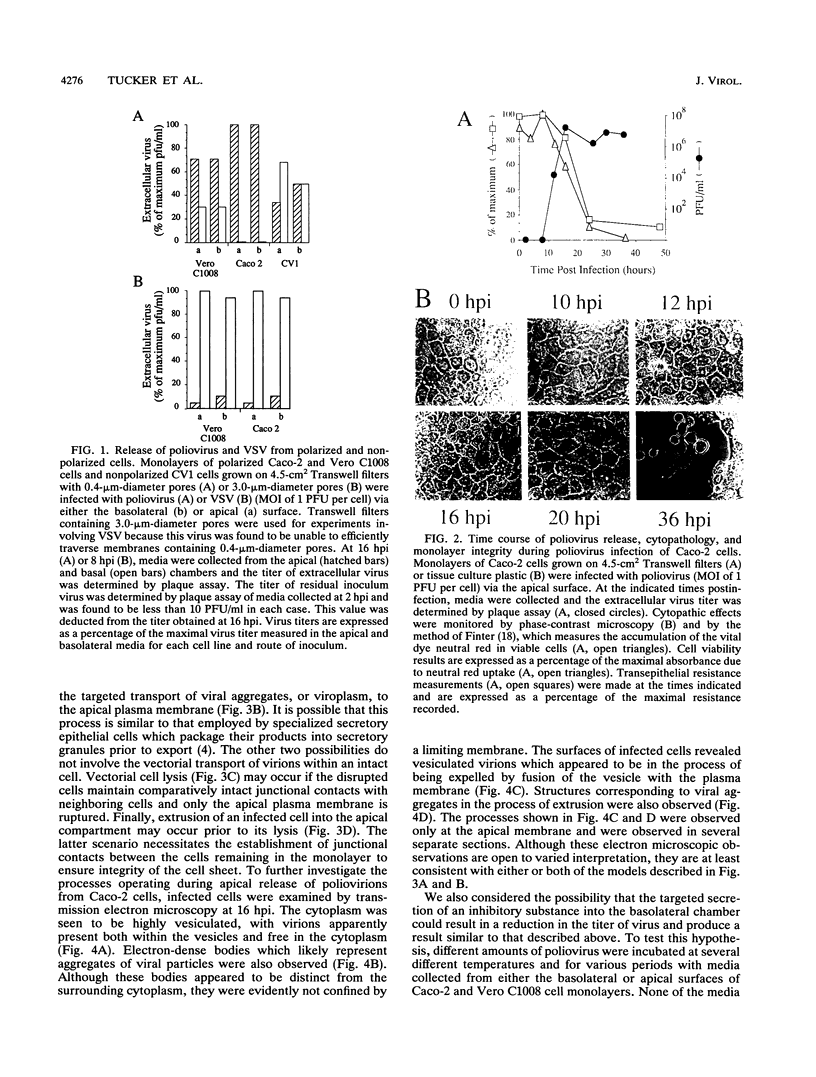
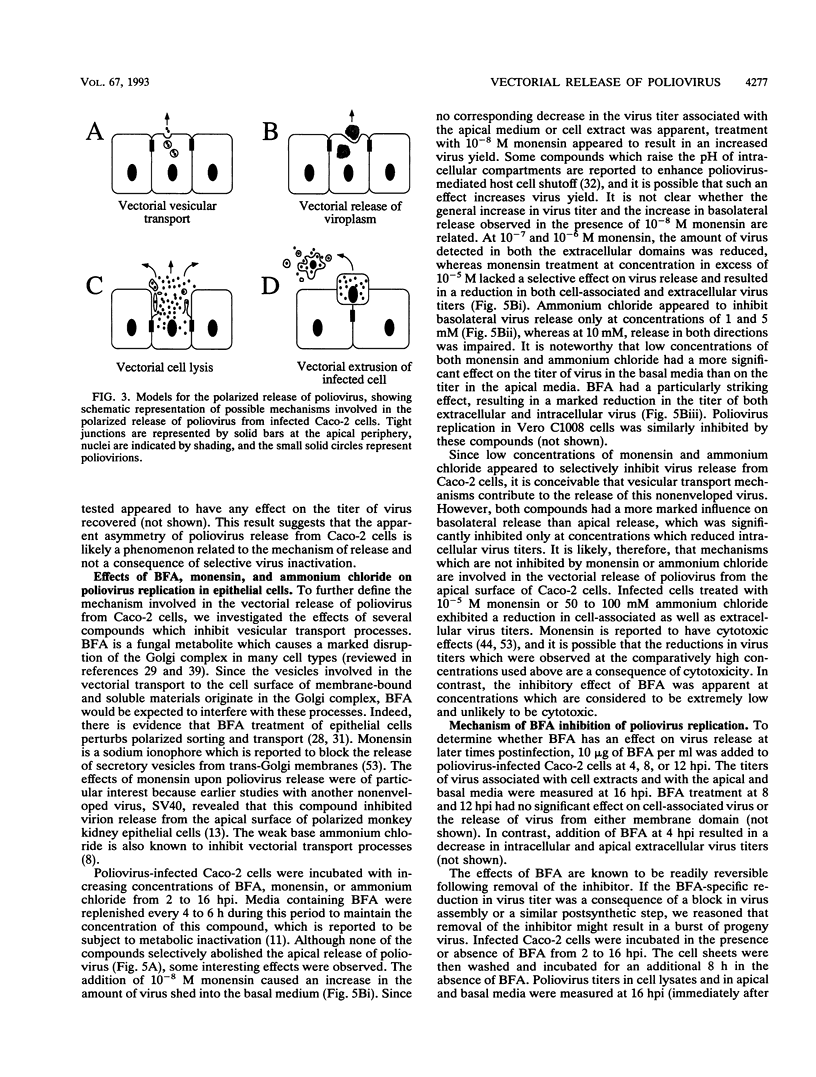
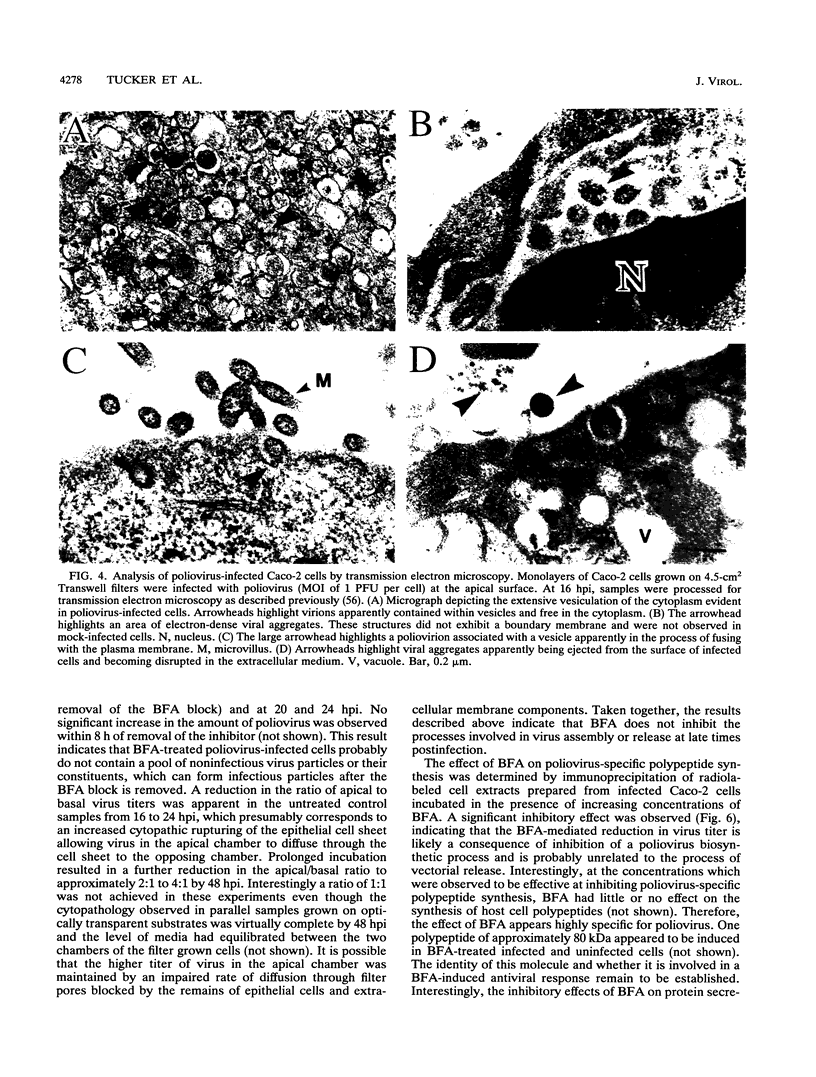
Polarized epithelial cells represent the primary barrier to virus infection of the host, which must also be traversed prior to virus dissemination from the infected organism. Although there is considerable information available concerning the release of enveloped viruses from such cells, relatively little is known about the processes involved in the dissemination of nonenveloped viruses. We have used two polarized epithelial cell lines, Vero C1008 (African green monkey kidney epithelial cells) and Caco-2 (human intestinal epithelial cells), infected with poliovirus and investigated the process of virus release. Release of poliovirus was observed to occur almost exclusively from the apical cell surface in Caco-2 cells, whereas infected Vero C1008 cells exhibited nondirectional release. Structures consistent with the vectorial transport of virus contained within vesicles or viral aggregates were observed by electron microscopy. Treatment with monensin or ammonium chloride partially inhibited virus release from Caco-2 cells. No significant cell lysis was observed at the times postinfection when extracellular virus was initially detected, and transepithelial resistance and vital dye uptake measurements showed only a moderate decrease. Brefeldin A was found to significantly and specifically inhibit poliovirus biosynthetic processes by an as yet uncharacterized mechanism. The vectorial release of poliovirus from the apical (or luminal) surface of human intestinal epithelial cells has significant implications for viral pathogenesis in the human gut.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODIAN D. Poliovirus in chimpanzee tissues after virus feeding. Am J Hyg. 1956 Sep;64(2):181–197. doi: 10.1093/oxfordjournals.aje.a119833. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987 Sep;160(1):220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Wolff D. A. Virus replication, cytopathology, and lysosomal enzyme response of mitotic and interphase Hep-2 cells infected with poliovirus. J Virol. 1973 Apr;11(4):565–574. doi: 10.1128/jvi.11.4.565-574.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Shimshick E. J., Yin F. H. Association of the polioviral RNA polymerase complex with phospholipid membranes. J Virol. 1976 Aug;19(2):457–466. doi: 10.1128/jvi.19.2.457-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Contreras R. G., Gonzalez-Mariscal L. Development and alteration of polarity. Annu Rev Physiol. 1989;51:785–795. doi: 10.1146/annurev.ph.51.030189.004033. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Matsuoka Y., Compans R. W. Assembly and polarized release of Punta Toro virus and effects of brefeldin A. J Virol. 1991 Mar;65(3):1427–1439. doi: 10.1128/jvi.65.3.1427-1439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P., Banfield B. W., Tufaro F. Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J Virol. 1991 Apr;65(4):1893–1904. doi: 10.1128/jvi.65.4.1893-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Brando L. V., Compans R. W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989 May;63(5):2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Srinivas R. V. Protein sorting in polarized epithelial cells. Curr Top Microbiol Immunol. 1991;170:141–181. doi: 10.1007/978-3-642-76389-2_5. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- Dunnebacke T. H., Levinthal J. D., Williams R. C. Entry and release of poliovirus as observed by electron microscopy of cultured cells. J Virol. 1969 Oct;4(4):505–513. doi: 10.1128/jvi.4.4.505-513.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstraunthaler G. J. Epithelial cells in tissue culture. Ren Physiol Biochem. 1988 Jan-Feb;11(1-2):1–42. doi: 10.1159/000173147. [DOI] [PubMed] [Google Scholar]
- Guinea R., Carrasco L. Effects of fatty acids on lipid synthesis and viral RNA replication in poliovirus-infected cells. Virology. 1991 Nov;185(1):473–476. doi: 10.1016/0042-6822(91)90802-i. [DOI] [PubMed] [Google Scholar]
- Guinea R., Carrasco L. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 1990 Jun;9(6):2011–2016. doi: 10.1002/j.1460-2075.1990.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Steele R. E. Factors affecting the differentiation of epithelial transport and responsiveness to hormones. Fed Proc. 1984 May 15;43(8):2221–2224. [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Hubbard A. L., Stieger B., Bartles J. R. Biogenesis of endogenous plasma membrane proteins in epithelial cells. Annu Rev Physiol. 1989;51:755–770. doi: 10.1146/annurev.ph.51.030189.003543. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Whitney J. A., Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991 Nov 1;67(3):617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- Irurzun A., Perez L., Carrasco L. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology. 1992 Nov;191(1):166–175. doi: 10.1016/0042-6822(92)90178-r. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu E. S., Ou J. H., Lee A. S. Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J Biol Chem. 1992 Apr 5;267(10):7128–7133. [PubMed] [Google Scholar]
- Low S. H., Wong S. H., Tang B. L., Tan P., Subramaniam V. N., Hong W. Inhibition by brefeldin A of protein secretion from the apical cell surface of Madin-Darby canine kidney cells. J Biol Chem. 1991 Sep 25;266(27):17729–17732. [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro L. S., Rose H. M., Morgan C., Hsu K. C. Electron microscopic study of the development of simian virus 40 by use of ferritin-labeled antibodies. J Virol. 1967 Apr;1(2):384–399. doi: 10.1128/jvi.1.2.384-399.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Mumbauer S., Hoke G. M., Takatsuki A., Sarngadharan M. G. Brefeldin A inhibits the processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1991 Aug;7(8):707–712. doi: 10.1089/aid.1991.7.707. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Multiple targets for brefeldin A. Cell. 1991 Nov 1;67(3):449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science. 1956 Jun 29;123(3209):1151–1157. doi: 10.1126/science.123.3209.1151. [DOI] [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Siciński P., Rowiński J., Warchoł J. B., Jarzabek Z., Gut W., Szczygieł B., Bielecki K., Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990 Jan;98(1):56–58. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Takeda N., Kuhn R. J., Yang C. F., Takegami T., Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986 Oct;60(1):43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tamura G., Ando K., Suzuki S., Takatsuki A., Arima K. Antiviral activity of brefeldin A and verrucarin A. J Antibiot (Tokyo) 1968 Feb;21(2):160–161. doi: 10.7164/antibiotics.21.160. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tershak D. R. Association of poliovirus proteins with the endoplasmic reticulum. J Virol. 1984 Dec;52(3):777–783. doi: 10.1128/jvi.52.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. P., Compans R. W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. P., Melsen L. R., Compans R. W. Migration of polarized epithelial cells through permeable membrane substrates of defined pore size. Eur J Cell Biol. 1992 Aug;58(2):280–290. [PubMed] [Google Scholar]
- Tucker S. P., Thornton C. L., Wimmer E., Compans R. W. Bidirectional entry of poliovirus into polarized epithelial cells. J Virol. 1993 Jan;67(1):29–38. doi: 10.1128/jvi.67.1.29-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Effects of brefeldin A on the processing of viral envelope glycoproteins in murine erythroleukemia cells. J Biol Chem. 1991 May 15;266(14):9173–9179. [PubMed] [Google Scholar]
- Whealy M. E., Card J. P., Meade R. P., Robbins A. K., Enquist L. W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991 Mar;65(3):1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Simons K. Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1982;1(7):847–852. doi: 10.1002/j.1460-2075.1982.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]