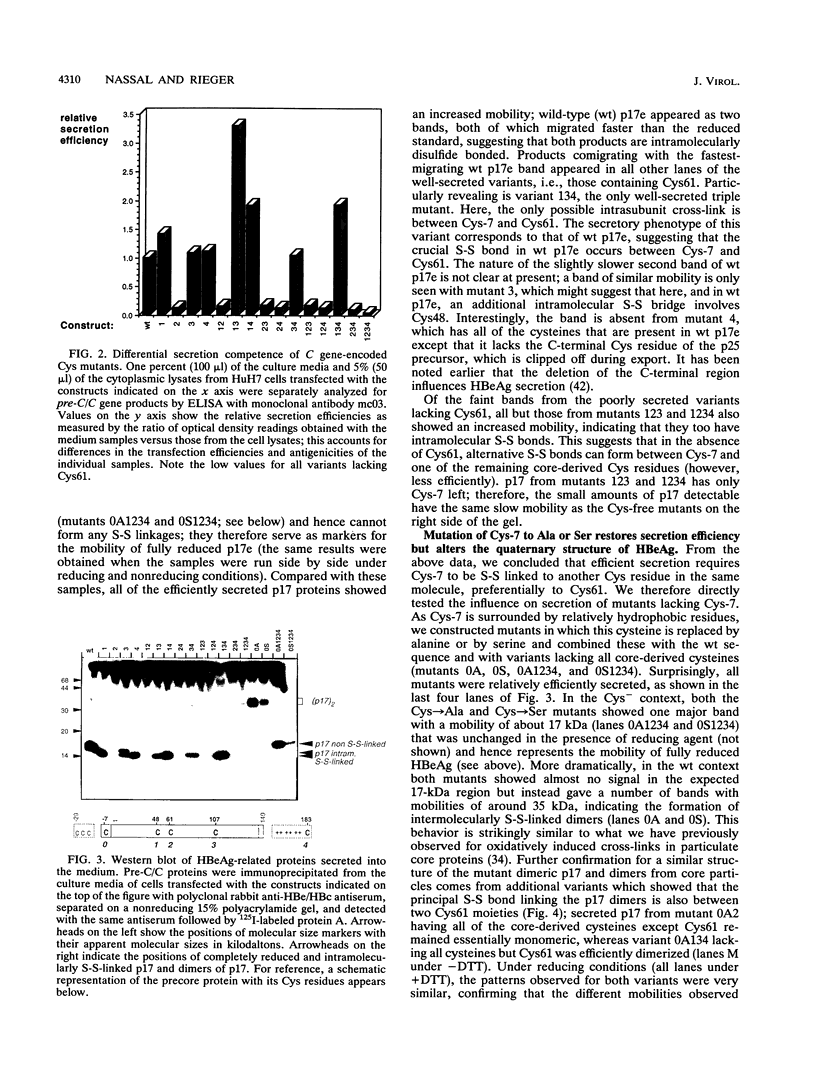
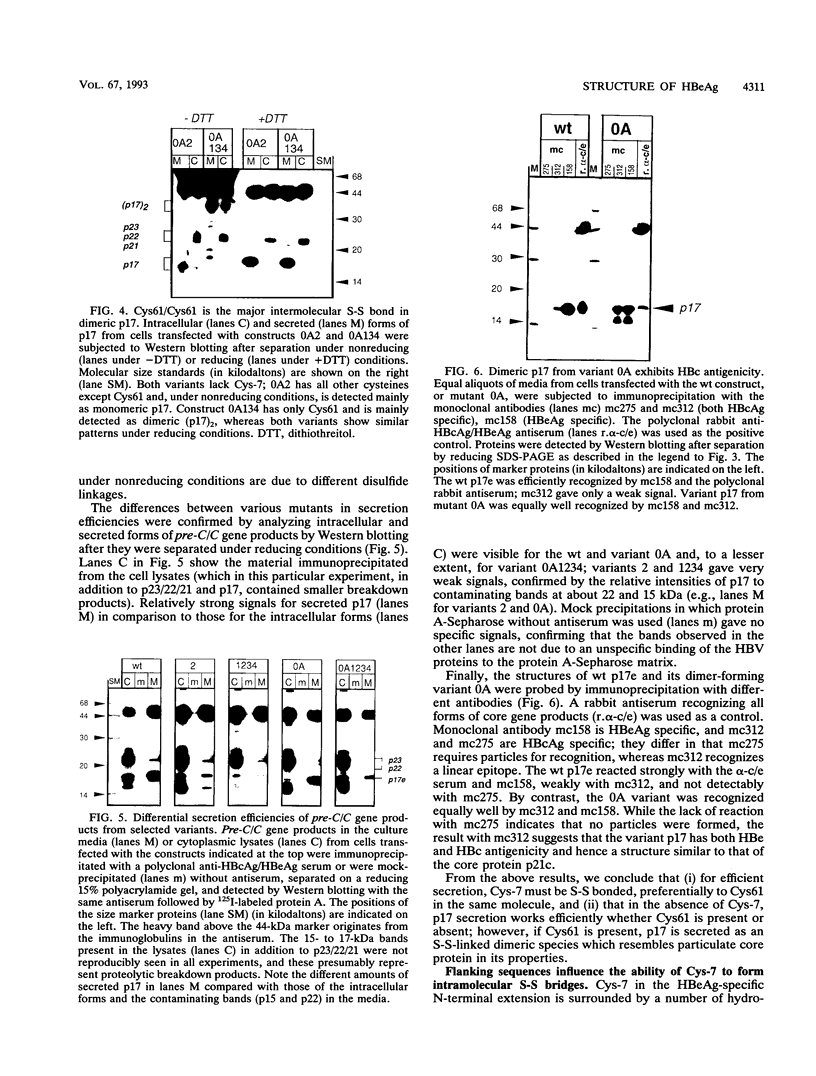
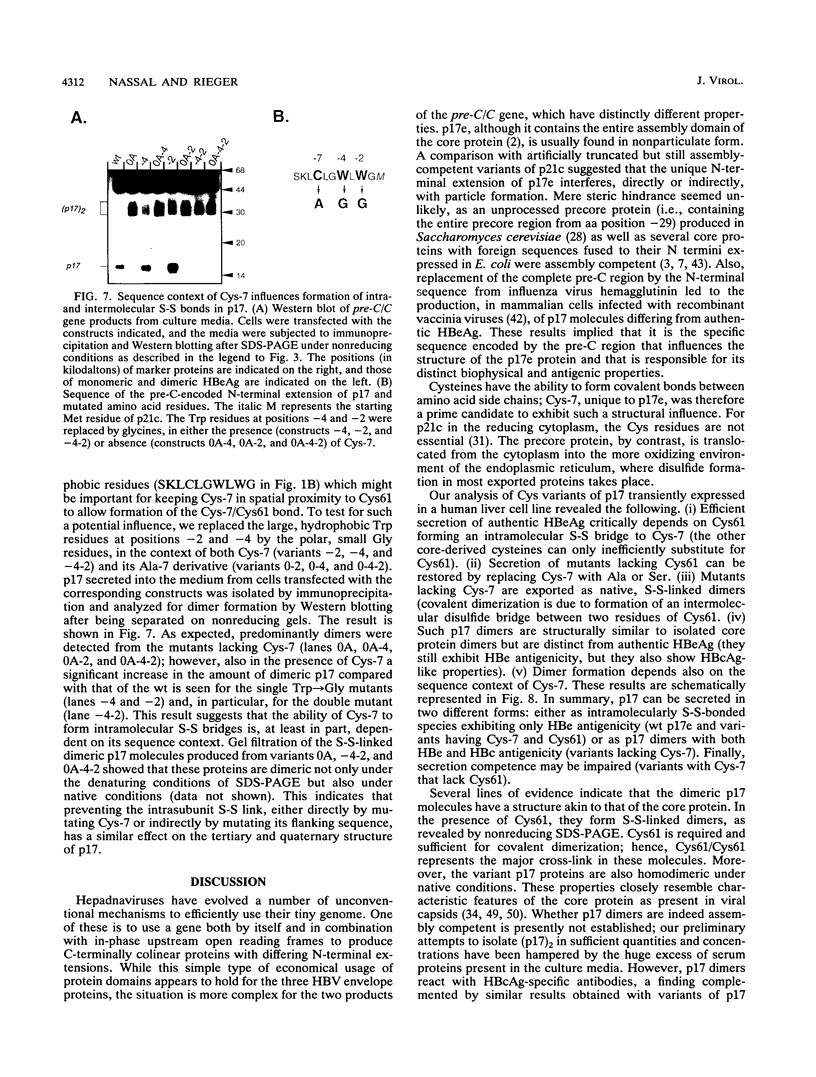
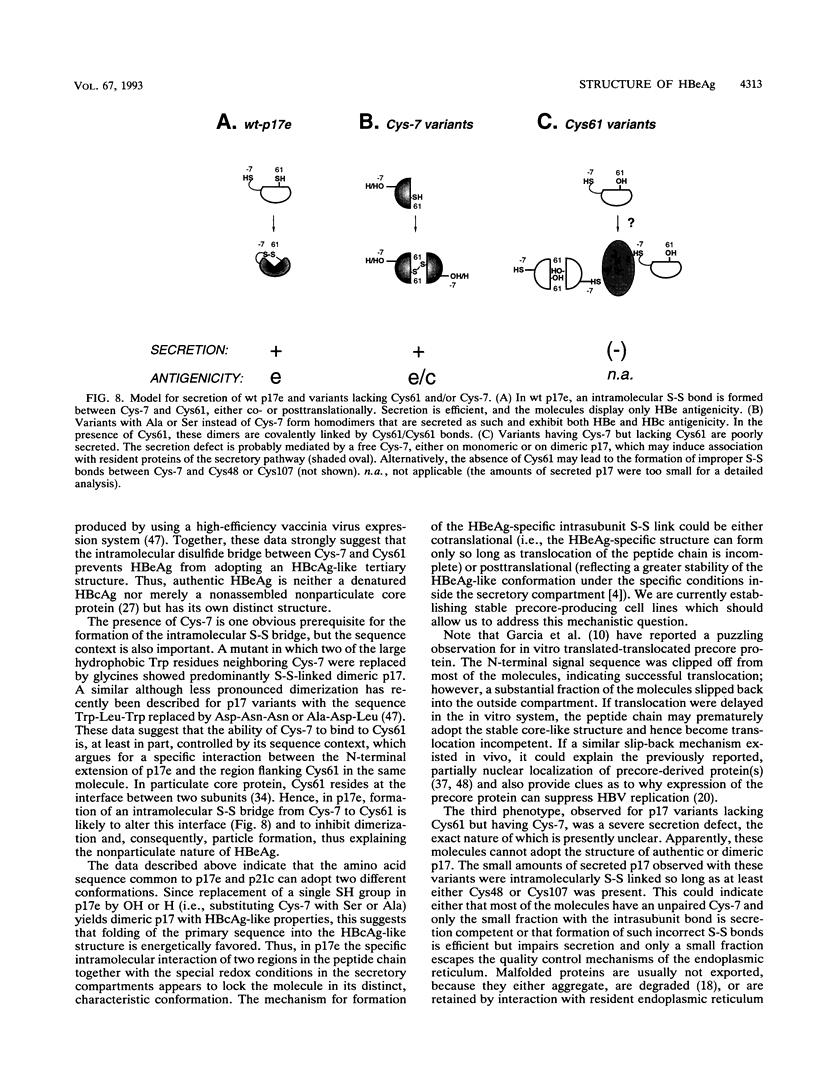
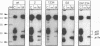Abstract
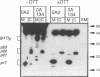
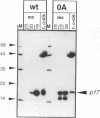
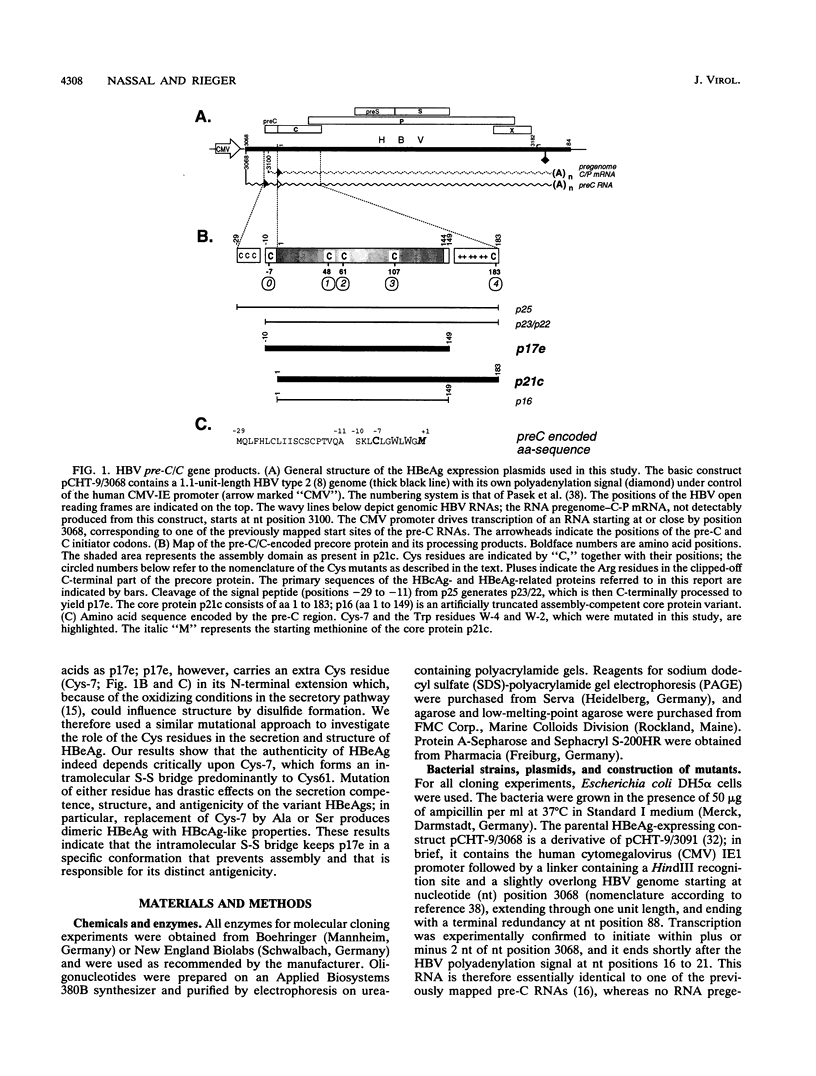
Hepatitis B virus, the prototypic member of the Hepadnaviridae, is a small enveloped DNA virus that replicates via reverse transcription. Efficient usage of its compact 3.2-kb genome is exemplified by the pre-C/C gene from which two proteins with largely overlapping primary sequences but distinctly different properties are synthesized: the self-assembling core protein p21c (hepatitis B core antigen [HbcAg]) and the secretory, nonparticulate protein p17e (hepatitis B e antigen [HbeAg]). Mature p17e carries a 10-amino-acid N-terminal extension with a Cys residue (Cys-7). Using transient transfection of a human liver cell line with constructs expressing wild-type p17 or a series of Cys mutants of p17, we show that Cys-7 forms an intramolecular S-S bond to Cys61, which in assembly-competent core proteins is available for intermolecular disulfide bonds between two neighboring subunits. Removal of the Cys-7/Cys61 bond by mutating either residue has differential effects: in the absence of Cys-7, secretion is relatively efficient and independent of Cys61; however, the molecules are exported as homodimers exhibiting both HBe and HBc antigenicity. In the absence of Cys61, the nonpaired Cys-7 interferes with secretion efficiency. The amino acid sequence flanking Cys-7 also contributes to the formation of the proper intramolecular S-S bond. These results suggest that the Cys-7/Cys61 bond imposes on p17e a conformation that is critical for its secretion and distinct biophysical and antigenic properties. This mechanism adds selective disulfide formation to the repertoire of hepatitis B virus for efficient use of its tiny genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C. M., Bet P., Milstein C., Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990 Oct 4;347(6292):485–487. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum F., Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990 Jul;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova G. P., Berzins I., Pushko P. M., Pumpen P., Gren E. J., Tsibinogin V. V., Loseva V., Ose V., Ulrich R., Siakkou H. Recombinant core particles of hepatitis B virus exposing foreign antigenic determinants on their surface. FEBS Lett. 1989 Dec 18;259(1):121–124. doi: 10.1016/0014-5793(89)81509-1. [DOI] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Carman W. F., Thomas H. C. The clinical significance of molecular variation within the hepatitis B virus genome. Hepatology. 1992 Jan;15(1):144–148. doi: 10.1002/hep.1840150124. [DOI] [PubMed] [Google Scholar]
- Chessler S. D., Byers P. H. Defective folding and stable association with protein disulfide isomerase/prolyl hydroxylase of type I procollagen with a deletion in the pro alpha 2(I) chain that preserves the Gly-X-Y repeat pattern. J Biol Chem. 1992 Apr 15;267(11):7751–7757. [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., Milanesi G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J Virol. 1989 Nov;63(11):4645–4652. doi: 10.1128/jvi.63.11.4645-4652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther S., Meisel H., Reip A., Miska S., Krüger D. H., Will H. Frequent and rapid emergence of mutated pre-C sequences in HBV from e-antigen positive carriers who seroconvert to anti-HBe during interferon treatment. Virology. 1992 Mar;187(1):271–279. doi: 10.1016/0042-6822(92)90315-g. [DOI] [PubMed] [Google Scholar]
- Hatton T., Zhou S., Standring D. N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in viral replication. J Virol. 1992 Sep;66(9):5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle J. H., Dusheiko G. M., Seeff L. B., Jones E. A., Waggoner J. G., Bales Z. B. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med. 1981 Jun;94(6):744–748. doi: 10.7326/0003-4819-94-6-744. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990 Oct;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loriot M. A., Marcellin P., Bismuth E., Martinot-Peignoux M., Boyer N., Degott C., Erlinger S., Benhamou J. P. Demonstration of hepatitis B virus DNA by polymerase chain reaction in the serum and the liver after spontaneous or therapeutically induced HBeAg to anti-HBe or HBsAg to anti-HBs seroconversion in patients with chronic hepatitis B. Hepatology. 1992 Jan;15(1):32–36. doi: 10.1002/hep.1840150107. [DOI] [PubMed] [Google Scholar]
- MacKay P., Lees J., Murray K. The conversion of hepatitis B core antigen synthesized in E coli into e antigen. J Med Virol. 1981;8(4):237–243. doi: 10.1002/jmv.1890080404. [DOI] [PubMed] [Google Scholar]
- Magnius L. O., Espmark J. A. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J Immunol. 1972 Nov;109(5):1017–1021. [PubMed] [Google Scholar]
- Manzin A., Menzo S., Bagnarelli P., Varaldo P. E., Bearzi I., Carloni G., Galibert F., Clementi M. Sequence analysis of the hepatitis B virus pre-C region in hepatocellular carcinoma [HCC] and nontumoral liver tissues from HCC patients. Virology. 1992 Jun;188(2):890–895. doi: 10.1016/0042-6822(92)90548-4. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Milich D. R. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988 Dec;9(12):380–386. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- Miyanohara A., Imamura T., Araki M., Sugawara K., Ohtomo N., Matsubara K. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: synthesis of two polypeptides translated from different initiation codons. J Virol. 1986 Jul;59(1):176–180. doi: 10.1128/jvi.59.1.176-180.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Nassal M. Conserved cysteines of the hepatitis B virus core protein are not required for assembly of replication-competent core particles nor for their envelopment. Virology. 1992 Sep;190(1):499–505. doi: 10.1016/0042-6822(92)91242-m. [DOI] [PubMed] [Google Scholar]
- Nassal M., Junker-Niepmann M., Schaller H. Translational inactivation of RNA function: discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell. 1990 Dec 21;63(6):1357–1363. doi: 10.1016/0092-8674(90)90431-d. [DOI] [PubMed] [Google Scholar]
- Nassal M., Rieger A., Steinau O. Topological analysis of the hepatitis B virus core particle by cysteine-cysteine cross-linking. J Mol Biol. 1992 Jun 20;225(4):1013–1025. doi: 10.1016/0022-2836(92)90101-o. [DOI] [PubMed] [Google Scholar]
- Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992 Jul;66(7):4107–4116. doi: 10.1128/jvi.66.7.4107-4116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M. Total chemical synthesis of a gene for hepatitis B virus core protein and its functional characterization. Gene. 1988 Jun 30;66(2):279–294. doi: 10.1016/0378-1119(88)90364-2. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yeh C. T., Yen T. S. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989 Dec;63(12):5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Salfeld J., Pfaff E., Noah M., Schaller H. Antigenic determinants and functional domains in core antigen and e antigen from hepatitis B virus. J Virol. 1989 Feb;63(2):798–808. doi: 10.1128/jvi.63.2.798-808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Wasenauer G. The quaternary structure, antigenicity, and aggregational behavior of the secretory core protein of human hepatitis B virus are determined by its signal sequence. J Virol. 1991 Dec;65(12):6817–6825. doi: 10.1128/jvi.65.12.6817-6825.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel F., Moriarty A. M., Peterson D. L., Zheng J. A., Hughes J. L., Will H., Leturcq D. J., McGee J. S., Milich D. R. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992 Jan;66(1):106–114. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990 Mar 9;60(5):781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Magnius L. O., Harthus H. P., Noah M., Wahren B. Characterisation of a linear binding site for a monoclonal antibody to hepatitis B core antigen. J Med Virol. 1991 Apr;33(4):248–252. doi: 10.1002/jmv.1890330407. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Noah M., Magnius L. O. Human and murine B-cells recognize the HBeAg/beta (or HBe2) epitope as a linear determinant. Mol Immunol. 1991 Jul;28(7):719–726. doi: 10.1016/0161-5890(91)90114-y. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Kishimoto S., Ohori K., Yoshizawa H., Machida A., Ohnuma H., Tsuda F., Munekata E., Miyakawa Y., Mayumi M. Molecular heterogeneity of e antigen polypeptides in sera from carriers of hepatitis B virus. J Immunol. 1991 Nov 1;147(9):3156–3160. [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Wasenauer G., Köck J., Schlicht H. J. A cysteine and a hydrophobic sequence in the noncleaved portion of the pre-C leader peptide determine the biophysical properties of the secretory core protein (HBe protein) of human hepatitis B virus. J Virol. 1992 Sep;66(9):5338–5346. doi: 10.1128/jvi.66.9.5338-5346.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Q., Walter M., Standring D. N. Hepatitis B virus p25 precore protein accumulates in Xenopus oocytes as an untranslocated phosphoprotein with an uncleaved signal peptide. J Virol. 1992 Jan;66(1):37–45. doi: 10.1128/jvi.66.1.37-45.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Schödel F., Peterson D. L. The structure of hepadnaviral core antigens. Identification of free thiols and determination of the disulfide bonding pattern. J Biol Chem. 1992 May 5;267(13):9422–9429. [PubMed] [Google Scholar]
- Zhou S., Standring D. N. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10046–10050. doi: 10.1073/pnas.89.21.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]