Abstract
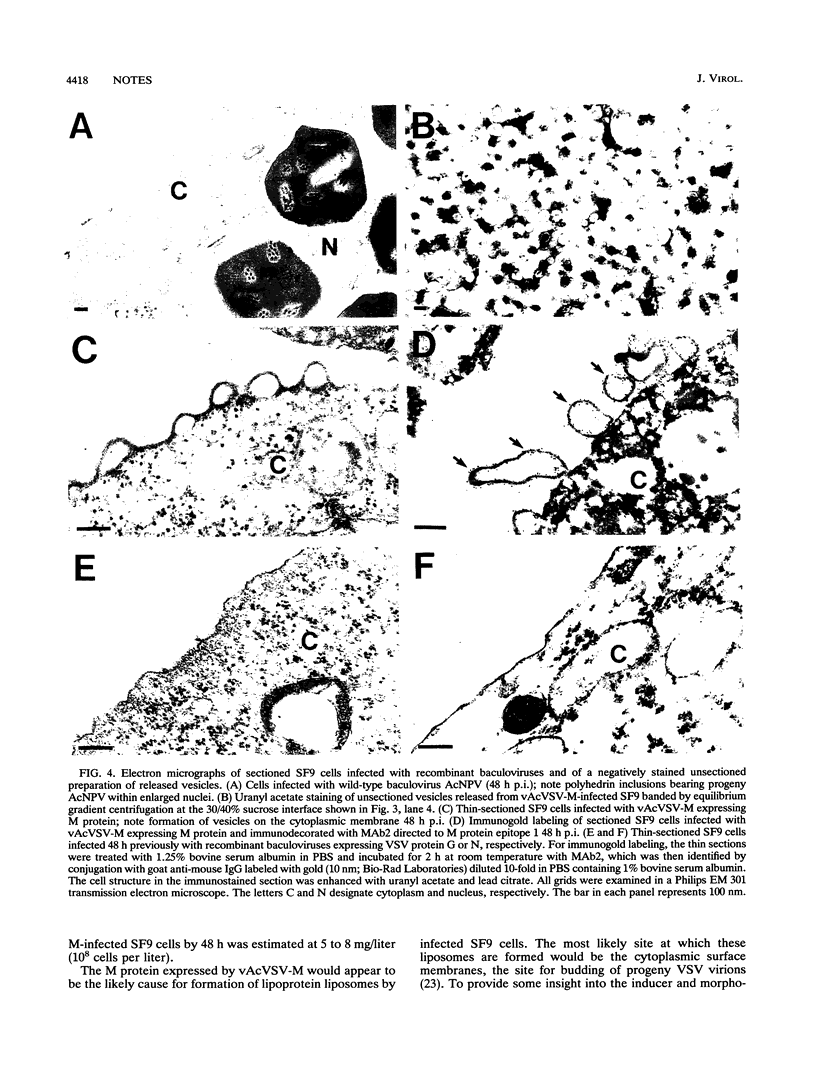
The matrix (M) protein of vesicular stomatitis virus (VSV) has been found to promote assembly and budding of virions as well as down-regulating of VSV transcription. Large quantities of M protein can be produced in insect cells infected with recombinant baculovirus expressing the VSV M gene under control of the polyhedrin promoter. Analysis by pulse-chase experiments and density gradient centrifugation revealed that the [35S]methionine-labeled M protein synthesized in insect cells is released into the extracellular medium in association with lipid vesicles (liposomes). Electron microscopy and immunogold labeling showed that M protein expressed in insect cells induced the formation on plasma membrane of vesicles containing M protein, which are released from the cell surface in the form of liposomes. The baculovirus vector itself or recombinants expressing VSV glycoprotein (G) or nucleocapsid (N) protein did not produce the formation of vesicles in infected cells. The baculovirus-expressed M protein retains biological activity as demonstrated by its capacity to inhibit transcription when reconstituted with VSV nucleocapsids in vitro. These data suggest that M protein has the capacity to associate with the plasma membrane of infected cells and, in so doing, causes evagination of the membrane to form a vesicle which is released from the cell. This observation leads to the postulate, which requires further proof, that the VSV M protein can induce the formation and budding of liposomes from the cell membrane surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., McLeod D. A., Kang C. Y., Bishop D. H. Glycosylation is not required for the fusion activity of the G protein of vesicular stomatitis virus in insect cells. Virology. 1989 Apr;169(2):323–331. doi: 10.1016/0042-6822(89)90157-8. [DOI] [PubMed] [Google Scholar]
- Blondel D., Harmison G. G., Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990 Apr;64(4):1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J Virol. 1983 Jan;45(1):80–90. doi: 10.1128/jvi.45.1.80-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L. D., Rose J. K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993 Jan;67(1):407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Schubert M. Location of the binding domains for the RNA polymerase L and the ribonucleocapsid template within different halves of the NS phosphoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5655–5659. doi: 10.1073/pnas.84.16.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptur P. E., Rhodes R. B., Lyles D. S. Sequences of the vesicular stomatitis virus matrix protein involved in binding to nucleocapsids. J Virol. 1991 Mar;65(3):1057–1065. doi: 10.1128/jvi.65.3.1057-1065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Site-specific mutations in vectors that express antigenic and temperature-sensitive phenotypes of the M gene of vesicular stomatitis virus. J Virol. 1988 Oct;62(10):3729–3737. doi: 10.1128/jvi.62.10.3729-3737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L., Rasool N., Kang C. Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993 Jan;67(1):584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Li Y., Cannon P. M., Kim S., Kang C. Y. Chimeric gag-V3 virus-like particles of human immunodeficiency virus induce virus-neutralizing antibodies. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10527–10531. doi: 10.1073/pnas.89.21.10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Li Y., Kang C. Y. Expression of gag precursor protein and secretion of virus-like gag particles of HIV-2 from recombinant baculovirus-infected insect cells. Virology. 1990 Dec;179(2):874–880. doi: 10.1016/0042-6822(90)90159-o. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener J. R., Pal R., Barenholz Y., Wagner R. R. Influence of the peripheral matrix protein of vesicular stomatitis virus on the membrane dynamics of mixed phospholipid vesicles: fluorescence studies. Biochemistry. 1983 Apr 26;22(9):2162–2170. doi: 10.1021/bi00278a017. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]