Abstract
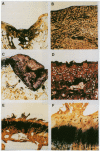
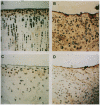
To determine if expression of specific proteoglycan epitopes distinguishes articular cartilage repair tissue from normal articular cartilage, we used seven monoclonal antibodies to examine normal articular cartilage and cartilage repair tissue from osteochondral defects 3.2 mm in diameter and 4.0 mm deep in the medial femoral condyles of 27 New Zealand white rabbits and seven cynomolgus monkeys. Following creation of the osteochondral defects, one limb of each animal was treated with cast immobilization while the other limb was treated with passive motion for two weeks. Rabbit knees were examined at eight (13 animals, 26 knees) and 36 weeks (14 animals, 28 knees) and monkey knees at eight weeks (seven animals, 14 knees) following surgery. Staining for six of the antibodies did not differ between repair cartilage and normal articular cartilage, but an antibody that recognizes atypical glycosaminoglycan structures in developing tissues (MAb 7D4) consistently distinguished repair cartilage from normal cartilage in rabbits and monkeys. Repair tissue consisting of hyaline toluidine blue-staining matrix containing chondrocytic cells uniformly showed strong 7D4 staining. In contrast, normal articular cartilage and fibrous repair tissue showed inconsistent weak 7D4 staining. At eight weeks following surgery, rabbit cartilage repair tissue stained more intensely for 7D4 than monkey cartilage repair tissue; in rabbits, cartilage repair tissue stained more intensely for 7D4 at eight weeks than at 36 weeks following surgery. Repair tissue staining for 7D4 did not differ between osteochondral defects treated with passive motion and those treated with immobilization in rabbits and monkeys. These results indicate that expression of a high level of proteoglycan epitope 7D4 distinguishes hyaline articular cartilage repair tissue from normal articular cartilage and fibrous cartilage repair tissue in the early stages of osteochondral healing, and that as hyaline articular cartilage repair tissue matures expression of 7D4 decreases. The ability to characterize repair cartilage proteoglycans with monoclonal antibodies may aid in the evaluation of the quality and maturity of cartilage repair tissue and thereby facilitate improvements in procedures for resurfacing joints.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckwalter J. A., Lohmander S. Operative treatment of osteoarthrosis. Current practice and future development. J Bone Joint Surg Am. 1994 Sep;76(9):1405–1418. doi: 10.2106/00004623-199409000-00019. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Woo S. L., Goldberg V. M., Hadley E. C., Booth F., Oegema T. R., Eyre D. R. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993 Oct;75(10):1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Caterson B., Griffin J., Mahmoodian F., Sorrell J. M. Monoclonal antibodies against chondroitin sulphate isomers: their use as probes for investigating proteoglycan metabolism. Biochem Soc Trans. 1990 Oct;18(5):820–823. doi: 10.1042/bst0180820. [DOI] [PubMed] [Google Scholar]
- Caterson B. Immunological aspects of markers of joint disease. J Rheumatol Suppl. 1991 Feb;27:19–23. [PubMed] [Google Scholar]
- Furukawa T., Eyre D. R., Koide S., Glimcher M. J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980 Jan;62(1):79–89. [PubMed] [Google Scholar]
- Ghadially F. N., Thomas I., Oryschak A. F., Lalonde J. M. Long-term results of superficial defects in articular cartilage: a scanning electron-microscope study. J Pathol. 1977 Apr;121(4):213–217. doi: 10.1002/path.1711210404. [DOI] [PubMed] [Google Scholar]
- Hall B. K. In vitro studies on the mechanical evocation of advenitious cartilage in the chick. J Exp Zool. 1968 Jul;168(3):283–305. doi: 10.1002/jez.1401680302. [DOI] [PubMed] [Google Scholar]
- Insall J. The Pridie debridement operation for osteoarthritis of the knee. Clin Orthop Relat Res. 1974 Jun;(101):61–67. [PubMed] [Google Scholar]
- Mitchell N., Shepard N. Effect of patellar shaving in the rabbit. J Orthop Res. 1987;5(3):388–392. doi: 10.1002/jor.1100050311. [DOI] [PubMed] [Google Scholar]
- Mitchell N., Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976 Mar;58(2):230–233. [PubMed] [Google Scholar]
- Salter R. B., Simmonds D. F., Malcolm B. W., Rumble E. J., MacMichael D., Clements N. D. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980 Dec;62(8):1232–1251. [PubMed] [Google Scholar]
- Shapiro F., Koide S., Glimcher M. J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993 Apr;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- Slater R. R., Jr, Bayliss M. T., Lachiewicz P. F., Visco D. M., Caterson B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995 May;38(5):655–659. doi: 10.1002/art.1780380513. [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Mahmoodian F., Schafer I. A., Davis B., Caterson B. Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem. 1990 Mar;38(3):393–402. doi: 10.1177/38.3.1689338. [DOI] [PubMed] [Google Scholar]