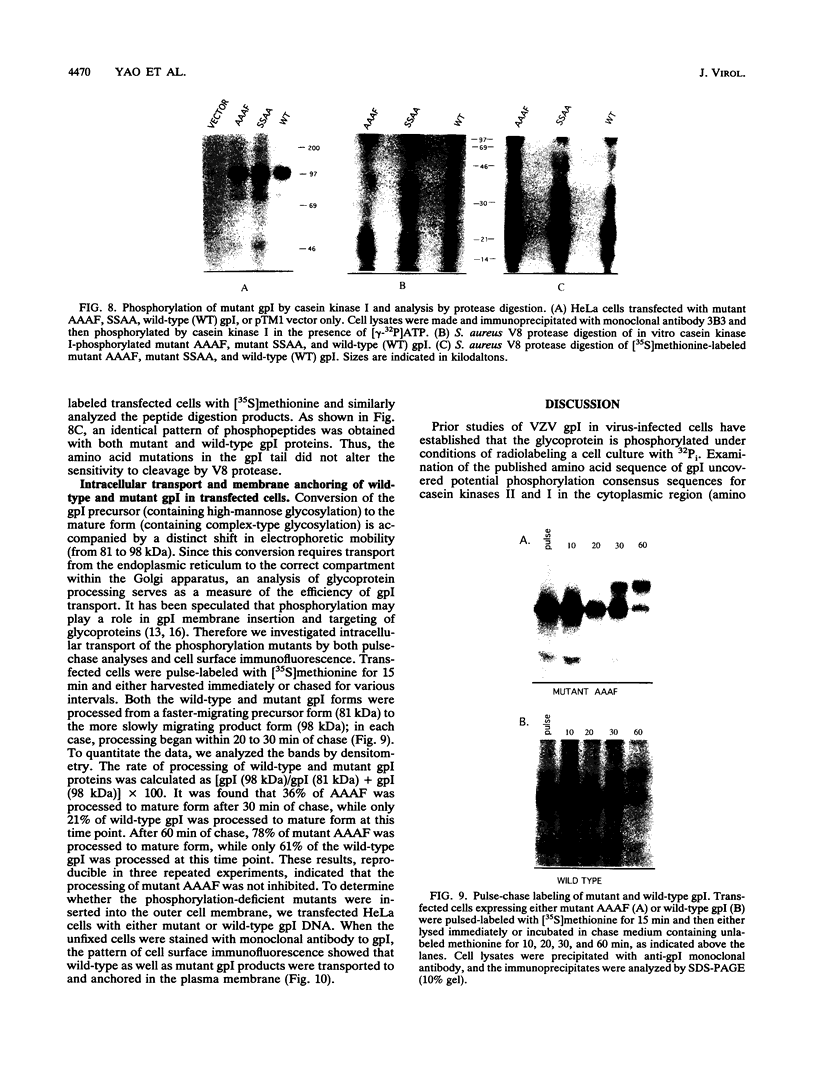
Abstract
Varicella-zoster virus (VZV) glycoprotein gpI, the homolog of herpes simplex virus gE, functions as a receptor for the Fc portion of immunoglobulin G. Like other cell surface receptors, this viral receptor is highly phosphorylated in cell culture. To identify the precise location of the cellular kinase-mediated phosphorylation, we generated a tailless deletion mutant and several point mutants which had altered serine and threonine residues within the cytoplasmic domain of gpI. The mutated and wild-type genes of gpI were transfected and expressed within a vaccinia virus-T7 polymerase transfection system in order to determine what effect these mutations had on the phosphorylation state of the protein in vivo and in vitro. Truncation of the cytoplasmic domain of gpI diminished the phosphorylation of gpI in vivo. Examination of the point mutants established that the major phosphorylation sequence of gpI was located between amino acids 593 and 598, a site which included four phosphorylatable serine and threonine residues. Phosphorylation analyses of the mutant and wild-type glycoproteins confirmed that gpI was a substrate for casein kinase II, with threonines 596 and 598 being critical residues. Although the mutant glycoproteins were phosphorylated by casein kinase I, protease V8 partial digestion profiles suggested that casein kinase II exerted the major effect. Thus, these mutagenesis studies demonstrated that the gpI cytoplasmic sequence Ser-Glu-Ser-Thr-Asp-Thr was phosphorylated in mammalian cells in the absence of any other herpesvirus products. Since the region defined by transfection was consistent with results obtained with in vitro phosphorylation by casein kinase II, we propose that VZV gpI is a physiologic substrate for casein kinase II. Immunofluorescence and pulse-chase experiments demonstrated that the mutant glycoproteins were processed and transported to the outer cell membrane.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Corvera S., Czech M. P. Mechanism of insulin action on membrane protein recycling: a selective decrease in the phosphorylation state of insulin-like growth factor II receptors in the cell surface membrane. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7314–7318. doi: 10.1073/pnas.82.21.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Waters D. J., Edson C. M. Identification of the products of a varicella-zoster virus glycoprotein gene. J Gen Virol. 1985 Oct;66(Pt 10):2237–2242. doi: 10.1099/0022-1317-66-10-2237. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Poodry C. A., Schooley R. T., Waters D. J., Thorley-Lawson D. A. Varicella-zoster virus envelope glycoproteins: biochemical characterization and identification in clinical material. Virology. 1985 Aug;145(1):62–71. doi: 10.1016/0042-6822(85)90201-6. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Waters D. J. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987 Dec;161(2):599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Dubey L., Steinberg S. P., Sherman D., Gershon M. D., Gershon A. A. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J Virol. 1989 Oct;63(10):4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- Grose C., Jackson W., Traugh J. A. Phosphorylation of varicella-zoster virus glycoprotein gpI by mammalian casein kinase II and casein kinase I. J Virol. 1989 Sep;63(9):3912–3918. doi: 10.1128/jvi.63.9.3912-3918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Jones F., Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988 Aug;62(8):2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Litwin V., Jackson W., Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992 Jun;66(6):3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V., Sandor M., Grose C. Cell surface expression of the varicella-zoster virus glycoproteins and Fc receptor. Virology. 1990 Sep;178(1):263–272. doi: 10.1016/0042-6822(90)90402-d. [DOI] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Grose C. Varicella zoster virus glycoprotein gpI is selectively phosphorylated by a virus-induced protein kinase. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8967–8971. doi: 10.1073/pnas.83.23.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Shin J., Doyle C., Yang Z., Kappes D., Strominger J. L. Structural features of the cytoplasmic region of CD4 required for internalization. EMBO J. 1990 Feb;9(2):425–434. doi: 10.1002/j.1460-2075.1990.tb08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Umphress J. L., Tuazon P. T., Chen C. J., Traugh J. A. Determinants on simian virus 40 large T antigen are important for recognition and phosphorylation by casein kinase I. Eur J Biochem. 1992 Jan 15;203(1-2):239–243. doi: 10.1111/j.1432-1033.1992.tb19852.x. [DOI] [PubMed] [Google Scholar]
- Yao Z., Jackson W., Forghani B., Grose C. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J Virol. 1993 Jan;67(1):305–314. doi: 10.1128/jvi.67.1.305-314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Jones D. H., Grose C. Site-directed mutagenesis of herpesvirus glycoprotein phosphorylation sites by recombination polymerase chain reaction. PCR Methods Appl. 1992 Feb;1(3):205–207. doi: 10.1101/gr.1.3.205. [DOI] [PubMed] [Google Scholar]