Abstract
After vascular injury, a cascade of serine protease activations leads to the conversion of the soluble fibrinogen molecule into fibrin. The fibrin monomers then polymerize spontaneously and noncovalently to form a fibrin gel. The primary interaction of this polymerization reaction is between the newly exposed N-terminal Gly-Pro-Arg sequence of the α chain of one fibrin molecule and the C-terminal region of a γ chain of an adjacent fibrin(ogen) molecule. In this report, the polymerization pocket has been identified by determining the crystal structure of a 30-kDa C-terminal fragment of the fibrin(ogen) γ chain complexed with the peptide Gly-Pro-Arg-Pro. This peptide mimics the N terminus of the α chain of fibrin. The conformational change in the protein upon binding the peptide is subtle, with electrostatic interactions primarily mediating the association. This is consistent with biophysical experiments carried out over the last 50 years on this fundamental polymerization reaction.
Keywords: fibrinogen, blood coagulation
Upon vascular injury, a series of protease activations culminates in the generation of thrombin, which mediates conversion of fibrinogen to fibrin by minor proteolysis (1). The resulting fibrin monomers polymerize spontaneously and noncovalently, forming two-stranded protofibrils that aggregate and form branches, creating a fibrin gel network. The fibrin polymers are stabilized further by the action of factor XIIIa, a transglutaminase that introduces covalent crosslinks into the network (2). The fibrin gel, together with activated platelets and other components of the blood coagulation system, forms a clot that staunches blood loss while the slower processes of tissue repair proceed. Disruption of this delicately balanced system can lead to bleeding or thrombotic disorders.
Fibrinogen is a 340-kDa multichain protein composed of two α, two β, and two γ chains that are interconnected by 29 disulfide bonds. In electron micrographs, fibrinogen (and the fibrin molecules comprising the initial protofibrils) appears as a trinodular structure (3). The N-terminal regions of the six chains are joined by five disulfide bonds between the (αβγ) half-molecules. This region constitutes the central nodule of the molecule. The three chains of each (αβγ) half-molecule extend outward in a symmetric fashion, forming two coiled-coil structures that terminate in globular domains in the distal two nodules. These distal nodules contain the C-terminal regions of the β and γ chains of the protein (4, 5).
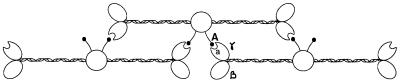
Fibrinogen is cleaved by thrombin, resulting in the release of short peptides from the N termini of the α and β chains. This creates two new N termini that are designated the “A” and “B” polymerization sites, respectively (6). The α chain is cleaved first, leading to a fibrin protofibril with a half-staggered overlap between molecules. As the fibrils grow, new additional A sites bind to complementary “a” binding pockets on the γ chains in the distal nodules of adjacent molecules (Fig. 1). The “a” binding pocket has been localized to residues 337 to 379 of the γ chain (7), and close to Y363 (8). The growth of two-stranded protofibrils then proceeds until they reach a length of about 600 nm, at which point the protofibrils aggregate to form thicker, branched fibers (9). The β chain is cleaved by thrombin primarily during the extension of the protofibrils, leading to the binding of its new N terminus to the as-yet-unidentified complementary “b” pocket. This enhances the rate and extent of lateral association of protofibrils (10). Cleavage of the α chain alone by proteases such as batroxobin, however, also leads to gel formation (11), indicating that the initial association of the complementary “a” and A sites is sufficient to promote clot formation.
Figure 1.
Simplified model of interactions between adjacent fibrin chains at the beginning of the polymerization reaction. Thrombin cleaves the α chain N terminus, creating a new N terminus (the A site) beginning with the sequence Gly-Pro-Arg. The A site binds to the complementary “a” polymerization pocket in the γ chain during the alignment of the fibrin protofibrils.
Short peptides mimicking the α-chain A sequence of GPRVV… will bind to the “a” polymerization pocket in the γ chain (8, 12–14). The peptides GPRP and GPRPamide bind even more tightly to the fibrinogen “a” site than does a peptide corresponding to the α chain native sequence GPRV (15). The complex between GPRP and fibrinogen has an increased resistance to degradation by plasmin (16), and the polymerization reaction is depressed by the addition of excess peptide (11, 12). The binding of calcium also stabilizes fibrinogen against plasmin digestion (17).
The polymerization of fibrin monomers has been characterized extensively in biophysical and biochemical experiments over the last five decades (18–24). The polymerization reaction is fully reversible before the covalent crosslinking of the fibrin chains by factor XIIIa (25, 26). Fibrin polymerization is exothermic, unlike many polymerization and protein-association reactions, which are primarily entropy driven (27). The association and aggregation of fibrils appears to involve the formation of new hydrogen bonds between adjacent fibrin molecules (22). Fibrin is also a polar molecule, and the alignment of molecules within fibrinogen crystals can be enhanced by placing the crystals in a magnetic field (28). The rate of the polymerization reaction in vitro is strongly dependent upon the salt concentration of the medium (9). It is therefore apparent that relatively nonspecific, long-range electrostatic interactions between polar domains of adjacent fibrin monomers are important in the initial alignment of the molecules.
The C-terminal region of the γ chain of fibrinogen also is involved in other reactions that are important for the structure and function of the molecule. It contains a single calcium-binding site (29–31) that influences fibrin polymerization as well as fibrinolysis (17). This region of fibrinogen also appears to have a role in inflammatory pathways (32). The final 15 residues at the C-terminal end of the γ chain contain lysine and glutamine side chains that are crosslinked by factor XIIIa (33). These C-terminal residues are also involved in the binding of fibrinogen to the GPIIb/IIIa receptor on activated platelets (34).
To study the biological functions of fibrin, Doolittle and coworkers (35) have performed x-ray diffraction studies on fragment D, a proteolytic fragment of fibrin(ogen) that comprises most of the distal nodule. In an alternative approach, we have cloned, expressed in Pichia pastoris, and crystallized a 30-kDa C-terminal fragment from the γ chain. This fragment, designated rFbgγC30, consists of residues γ143–411. Addition of rFbgγC30 to a preparation of fibrinogen cleaved by batroxobin, which cleaves the α but not the β chains of fibrinogen, inhibited polymerization, indicating that the “a” polymerization pocket is present and functional in the recombinant γ chain fragment (unpublished work). Recently the structure of rFbgγC30 was solved at 2.1 Å resolution, leading to the identification of the calcium-binding site (31). In this manuscript the 2.1 Å resolution crystal structure of a complex between rFbgγC30 and the peptide GPRP is presented, leading to the identification of the polymerization pocket. These data provide a clear picture of the specific molecular interactions mediating the first critical step of fibrin polymerization.
MATERIALS AND METHODS
Crystallization.
Crystals were grown from sitting drops containing 12% polyethylene glycol 8000 (Fluka), 70 mM CaCl2, 0.1 M Mes at pH 6.0, and 0.02% NaN3, at room temperature. Protein concentration was 30 mg/ml, and 2 μl of protein was added to 2 μl of well solution in crystal growth chambers purchased from Charles Supper Co. (Natick, MA). The calcium concentration was suggested by reference to a report of crystallization conditions for fibrinogen fragment D (35).
Data Collection and Refinement.
The crystal was soaked overnight in the original solution plus 0.01 M Gly-Pro-Arg-Pro (GPRP; Sigma). The crystal was then back-soaked in the original well solution for 10 min, and data were collected using CuKα x-rays at room temperature on a Rigaku R-axis IIc area detector system. The Rigaku process software was used to obtain integrated intensities and to scale the data. The resulting structure was isomorphous with the P21 structure solved by Yee et al. (31), and the peptide GPRP was built into an Fo − Fc difference density map. Calculation of maps and fitting of the protein and peptide models were carried out using xtalview (36), and atomic positions and B factors were refined using xplor (37). Ten percent of the reflections were omitted from the refinement and used to monitor Rfree as refinement progressed. As in the P21 structure reported by Yee et al. (31), the electron density map beyond Leu-392 was not clear enough to fit additional residues. Mass spectrometry showed that the protein solutions used to grow crystals of rFbgγC30 contained a mixture of populations proteolyzed to different extents at the C terminus. The use of protease inhibitors alleviated, but did not eliminate, this problem (data not shown). Data collection and refinement statistics are presented in Table 1. Difference density maps showed that there is a single unique binding site for the peptide.
Table 1.
Data collection and refinement statistics
| Data collection | |
| Space group | P21 |
| a,* Å | 37.33 |
| b,* Å | 68.20 |
| c,* Å | 47.60 |
| β, ° | 105.02 |
| Resolution, Å | 68.2–2.0 |
| Rsym(I),* % | 5.40 |
| Completeness, % | 76.9 |
| Refinement | |
| Resolution, Å | 10.0–2.0 |
| No. of reflections | 12,275 |
| No. of protein + peptide atoms | 2,034 |
| No. of solvent molecules | 117 |
| No. of calcium ions | 1 |
| R value (free R),† % | 18.6 (28.2) |
| RMSD bond lengths,‡ Å | 0.010 |
| RMSD bond angles,‡ ° | 1.903 |
| Protein mean B/max B, Å2 | 23.5/50.2 |
| GPRP mean B. Å2 | 37.4 |
| Solvent mean B/max B, Å2 | 34.9/61.1 |
Rsym = ΣhklΣi |Ii(hkl) − 〈I(hkl)〉|Σhkl〈I(hkl)〉, where Ii(hkl) is the ith measurement and 〈I(hkl)〉 is the weighted mean of all measurements of I(hkl).
Crystallographic R value = Σhkl ∥Fo| − |Fc∥/Σhkl|Fo| with 90% of the data used for refinement. Free R value is the R value based on 10% of the native data withheld from refinement.
RMSD bond lengths, bond angles, dihedral angles, and improper torsion angles are the rms deviations from the standard geometry used in xplor (37).
RESULTS AND DISCUSSION
Specific Interactions in the Polymerization Pocket.
The conformational change of the γ chain is localized and subtle upon binding GPRP, with very little change in the protein backbone (Fig. 2). However, the side chains of a few amino acid residues in the “a” polymerization pocket move several angstroms to accommodate binding of the peptide. These residues include D364, R375, H340, and Q329, and their movement results in the rearrangement of a network of hydrogen bonds and salt links that stabilize the “a” polymerization pocket (Fig. 3). In the complex, the side chain of Q329 moves toward the solvent, enlarging the binding pocket and hydrogen bonding to the peptide arginine side chain. The side chains of D330 and H340 shift position in a concerted movement that preserves their shared hydrogen bond. The hydrogen bond from the backbone carbonyl oxygen of H340 to a water molecule is replaced by a hydrogen bond to the peptide glycine N terminus. D364 moves slightly, breaking its salt link to R375 and forming a strong ionic interaction with the charged peptide N terminus. Y363 loses a hydrogen bond to a water molecule and moves to accommodate the arginine side chain of GPRP. In the uncomplexed structure, K338 and E323 form a salt link. When GPRP binds, the two residues shift slightly, allowing K338 to interact also with the C terminus of the peptide. The key interactions of the “a” site before and after complex formation are listed in Table 2. The location of the binding site is consistent with results of experiments using proteolytic fragments of fibrinogen (7, 29), and it includes Y363, which was previously localized to the polymerization pocket by a photolabeling study (8). In addition, several amino acids whose substitution is known to cause polymerization defects in patients are clustered at this site and interact directly with the peptide, indicating that this site is indeed the physiologically relevant “a” polymerization pocket. A cursory examination of this structure revealed that the calcium-binding site at residues 318–320 is adjacent to the “a” polymerization pocket, but none of the amino acids that ligand the calcium, i.e., D318, D320, F322, and G324, interact with the bound peptide (Fig. 4A).
Figure 2.
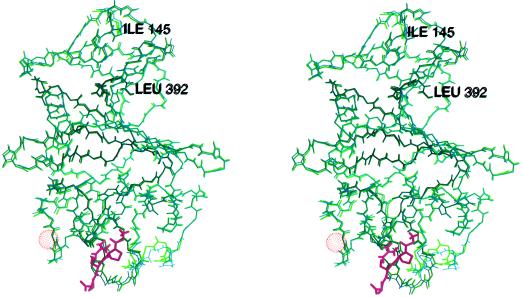
Stereoview of the superimposed backbones of the rFbgγC30 molecules in the peptide complexed (green) and uncomplexed (purple) structures. The peptide Gly-Pro-Arg-Pro is shown in magenta, and the van der Waals surface of the calcium atom is shown as an orange dot-surface. Because the first two residues are flexible, the superposition begins with the third residue, Ile-145.
Figure 3.
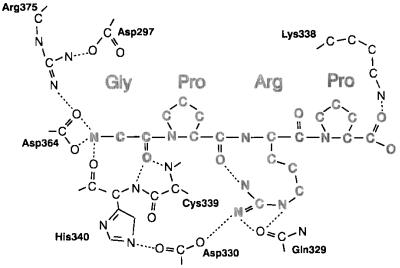
Schematic of interactions between GPRP and the protein. Hydrogen bonds and favorable ionic interactions are indicated by dotted lines. One of the terminal nitrogens of the arginine side chain is 3.26 Å away from the carbonyl oxygen of the first peptide proline, creating a weak hydrogen bond.
Table 2.
Binding pocket hydrogen bonds
| Atom | Partner (uncomplexed) | Partner (complexed) |
|---|---|---|
| D297(OD1) | (−) | R375(NH1) |
| D297(OD2) | R375(NH1,NH2), HOH487 | (−) |
| E323(OE1) | HOH480 | (−) |
| E323(OE2) | HOH480, K338(NZ) | K338(NZ) |
| N325(N) | HOH469 | (−) |
| Q329(NE2) | HOH415, HOH473 | (−) |
| Q329(OE1) | (−) | Arg-3(NE,NH2) |
| D330(OD1) | HOH415 | Arg-3(NH2) |
| D330(OD2) | H340(NE2) | H340(NE2) |
| K338(NZ) | E323(OE2) | E323(OE2), Pro-4(OT2) |
| C339(N) | HOH423 | Gly-1(O) |
| C339(O) | N337(N) | N337(N) |
| H340(N) | HOH423 | Gly-1(O) |
| H340(O) | HOH488 | Gly-1(N) |
| H343(ND1) | HOH421 | G366(O) |
| H343(NE2) | S332(OG) | S332(OG) |
| Y363(OH) | HOH556 | (−) |
| D364(OD1) | HOH415 | Gly-1(N) |
| D364(OD2) | HOH488,R375(NE,NH2) | Gly-1(N) |
| G366(O) | (−) | H343(ND1) |
| R375(NH2) | HOH408, D297(OD2), D364(OD2) | (−) |
| R375(NE) | D364(OD2) | (−) |
| R375(NH1) | HOH481, D297(OD2) | D297(OD1) |
| Arg-3(NH1) | N/A | Pro-3(O) |
| Pro-4(OT1) | N/A | HOH440 |
| Pro-4(OT2) | N/A | K338(NZ) |
Residues and atoms in GPRP peptide are shown in boldface type. N/A, not applicable.
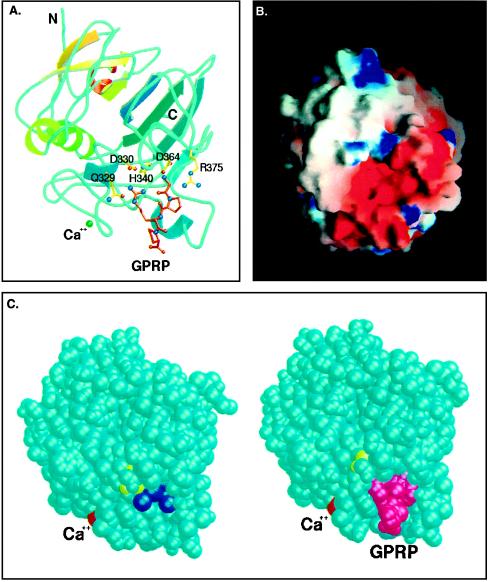
Figure 4.
(A) Model of rFbgγC30 with the peptide GPRP bound at the “a” polymerization pocket. The side chains of residues Q329, D330, H340, D364, and R375 are involved in interactions that stabilize the complex with the peptide. Figure created using molscript (38) and raster3d (39). (B) End-on view of the “a” polymerization pocket, colored according to the electrostatic potential computed using the program grasp (40). The high concentration of negatively charged residues at this site, as well as the depth and narrowness of the pocket, confer its specificity for the positively charged GPR sequence of the α chain of fibrin. The model is of the peptide-complexed structure of rFbgγC30, with the peptide removed to show the binding site more clearly. (C) Space-filling models of rFbgγC30 in the uncomplexed and peptide-complexed structures. The peptide GPRP (magenta) displaces seven tightly bound water molecules (dark blue). The calcium atom is colored red, and Y363, which was shown previously by Doolittle and coworkers (7) to be close to the binding site, is colored yellow. Figure was created using molscript (38) and raster3d (39).
The high-resolution structure of the primary polymerization, or “a” binding pocket, provides a clear picture of the specific amino acid interactions mediating the first step of fibrin polymerization. The unoccupied “a” binding pocket has a strongly negative electrostatic potential (Fig. 4B). After cleavage of the fibrinogen α chain by thrombin, the new N-terminal sequence of GPR in fibrin carries two positive charges. The interaction of these clusters of positive and negative charges plays an important role in the alignment of the rod-like fibrin monomers into half-staggered overlapping protofibrils. In the complex between rFbgγC30 and GPRP, both the positively charged N terminus and the arginine side chain are buried in the protein interior, thus strengthening these ionic interactions. Also, seven well defined water molecules, including several that are deep in the “a” polymerization pocket of rFbgγC30, are displaced upon binding of the GPRP peptide (Fig. 4C). Additional rearrangements of amino acids and solvent may well occur upon the association of the central and distal nodules of fibrin during polymerization. The predominance of ionic interactions in the binding of GPRP is consistent with experiments showing that the polymerization process involves formation of new hydrogen bonds between adjacent fibrin molecules (22). These specific interactions account for the importance of the free amino group of the peptide glycine. They also explain how the fibrinopeptide A region in fibrinogen would prevent the initiation of polymerization at the “a” site before thrombin cleavage. The negative charge of the α chain N terminus is reduced when the acidic fibrinopeptide A is removed by thrombin, thus enhancing the ionic interactions between the newly generated N terminus and the “a” polymerization site.
The existence of a critical histidine residue at the polymerization site(s) has been postulated, based upon the pH dependence of fibrin polymerization (19), and upon the uptake or release of protons measured when fibrin polymerization is monitored through a range of pH values (22, 41). At pH 6.8, the release of protons lagged behind the cleavage by thrombin of the α chain (41), suggesting that protons were released coincident with the polymerization reaction. There are two histidine residues at the “a” polymerization pocket, H340 and H343. H340 forms a hydrogen bond with D330 in both the uncomplexed and in the peptide-complexed structures. In the uncomplexed structure, the side chain of H340 also forms a hydrogen bond to a water molecule, which in turn forms a hydrogen bond to the carboxylate group of D364. H343 is at the internal apex of the polymerization pocket and is hydrogen-bonded to a water molecule and to the side chain of S332 in the uncomplexed structure. In the peptide complex this water molecule is expelled, allowing the side chain of H343 to become a proton acceptor in a new hydrogen bond to G366. Fibrin does not polymerize below pH 5.0. Clearly, the ionization of either or both of these histidine residues at lower pH values would affect the ionic interactions within the polymerization pocket, even though the histidine side chains do not hydrogen-bond directly to the GPRP peptide.
Nonproline cis Peptide Bond.
An unusual cis peptide bond between residues K338 and C339 allows the backbone nitrogen of C339 to form a hydrogen bond with the carbonyl oxygen at the peptide glycine (Fig. 5A). Residues 337–340 form a tight β-turn, with N337 having strained (φ,ψ) dihedral angles. It is interesting to note that strained backbone conformations, although quite rare in proteins, tend to be localized in regions of functional significance, e.g., at metal- or ligand-binding sites (42, 43). The folding of proteins in which proline residues following cis peptide bonds are replaced by alanine has been investigated recently (44–46), demonstrating that the native conformations of several proteins can accommodate the extra strain of a nonproline cis peptide bond, although the stability of the protein may decrease. The folding of a similar Pro-to-Ala mutant in human carbonic anhydrase II also has been demonstrated unequivocally by the solution of its crystal structure (47), which showed that the cis peptide bond is retained in the mutant. During the folding of these proteins, it is likely that the local formation of “native-like” structure in the vicinity of the cis peptide bond provides stabilizing interactions (such as hydrogen bonds, metal ligand binding, etc.) that compensate energetically for the backbone strain and direct the protein toward the native conformation. In the uncomplexed γ chain, N337 is involved in six hydrogen bonds, to the nearby residues F303, S306, K321, K338, C339, and to a water molecule. The chain reversal here is stabilized further by the side chain of H307, which forms hydrogen bonds with the backbones of residues 335 and 341.
Figure 5.
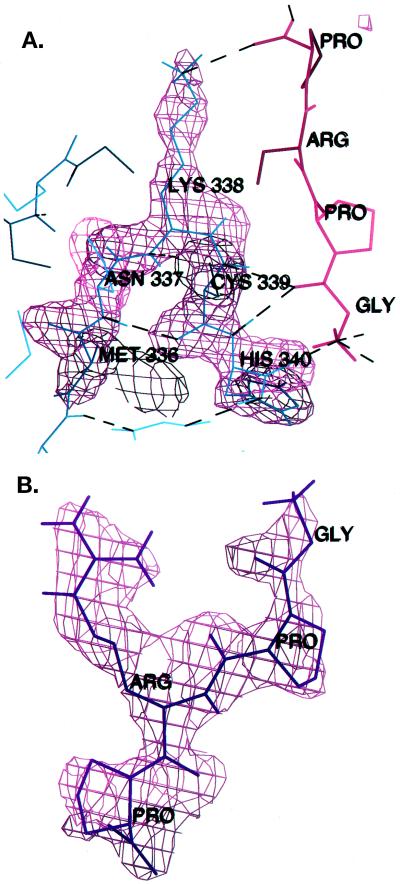
(A) Electron density is shown superimposed on the turn containing the cis peptide bond between K338 and C339 in the peptide-complexed structure. |Fo| − |Fc| omit maps were calculated after omitting all atoms of residues 336–340, and the map is contoured at 3σ. Hydrogen bonds are drawn as dashed lines. (B) The GPRP peptide was removed from the model and an |Fo| − |Fc| map was calculated and contoured at 3σ, then superimposed upon the peptide model.
Refinement of the original P21 structure (31) of the uncomplexed rFbgγC30 fragment was continued, to investigate the possibility of cis-trans isomerization at the K338-C339 peptide bond. This resulted in minor modifications of the coordinates and of some secondary structure assignments. Specifically, residues 336–340, which are part of a tight turn, were rebuilt with a cis peptide bond between K338 and C339. After positional and B-refinement, Rcryst dropped from 15.5 to 15.4, Rfree dropped from 22.7 to 20.6, and the fit of this region to the density improved markedly. In addition, residues 215 to 217 and 225 to 227 are no longer referred to as β strands, because some of the backbone dihedral angles are outside the range usually described as an extended β conformation.
Fig. 5A shows this region of the peptide-complexed structure with the electron density superimposed. The density is well defined, and the possibility of cis-trans isomerization at this site in the folded protein appears unlikely. The fit of the peptide GPRP to the electron density is shown in Fig. 5B. Several other specific interactions may be critical for the stabilization of this strained region. The disulfide bond between C326 and C339 anchors the position of C339. The backbone nitrogen of C339 hydrogen bonds to a tightly bound water molecule with a low temperature factor in the uncomplexed protein (B = 11.3 Å2). In the complex with GPRP, this water is replaced by the carbonyl oxygen of the peptide glycine and the hydrogen bonding network shifts somewhat. The hydrogen bond between S306 and N337 is replaced by hydrogen bonds between S306 and two water molecules. N337 and K338 are stabilized by hydrogen bonds to the backbone atoms of F303 and F304. The carbonyl oxygen of H340 hydrogen bonds to the peptide N terminus, which interacts in turn with D364. These interactions stabilize the protein structure as they strengthen the intermolecular association at the polymerization site. The precise alignment of residues in this extensive network of hydrogen bonds is consistent with the hypothesis of Herzberg and Moult (42) that strained regions tend to have well defined interatomic distances due to the extra hydrogen bonds and other stabilizing interactions that are necessary to counteract the strain in these regions. This “rigidity” then confers an evolutionary benefit upon the protein when the strained region is part of a binding or catalytic site where the precise alignment of residues is critical to the function of the protein.
Structure-Based Explanations of Dysfibrinogenemias.
Clinical studies have provided an extensive database of dysfibrinogenemias (48), most of which have been identified in routine assays of the blood of patients, followed by DNA sequence analysis of the three genes coding for fibrinogen. The mutants Q329R (48), D330Y (48), D330V (48), N337K (48), D364H (49), and R375G (48) result in defective polymerization and are part of the polymerization pocket. Q329 shifts position upon complex formation to accommodate the arginine side chain of the peptide. The substitution of an arginine for a glutamine at this site would block the peptide arginine-binding site in the mutant protein. D330 is hydrogen-bonded to H340 in both the complexed and uncomplexed structures. It forms a salt link to the peptide arginine in the GPRP complex. The substitution of this side chain by tyrosine or valine would clearly destabilize the interactions within the “a” polymerization pocket. The side chain of N337 points away from the polymerization pocket into the solvent. Although a lysine side chain could be accommodated here, this substitution could result in the loss of critical hydrogen bonds that stabilize the conformation of this strained region. The side chain of R375 lines the “a” pocket and preserves its structural integrity through a series of hydrogen bonds (Table 2). The mutation of this residue to glycine would result in an enlargement of the polymerization site and a rearrangement of hydrogen bonding among neighboring residues, thus precluding the formation of an effective complex with the α chain. The substitution D364H (49) would result in the loss of the salt link between this residue and the charged N terminus of the α chain.
The analysis of γ chain mutants in terms of patient pathology is complicated by several factors. Most of the patients carrying these defective fibrinogen molecules are heterozygotes; therefore fibrin polymerization proceeds with a mixture of normal and defective molecules. This will lead in many cases to altered fiber morphologies in the resulting fibrin gel, which may in turn affect the breakdown of clots by plasmin. Furthermore, the interactions of the defective fibrinogen molecules with other molecules in the procoagulant and fibrinolytic pathways may be altered in unpredictable ways. Thus, the disruption of the initial polymerization reaction may cause a bleeding defect in some cases, or thrombophilia in other cases when the mechanisms for clot dissolution are disrupted.
The lack of a crystal structure for fibrin(ogen) has long hampered structure-function studies aimed at understanding and modifying its various properties (50). The crystal structure of the γ chain C-terminal region complexed with a peptide analog of the activated fibrin α chain now can provide a basis for structure-based drug design, where the goal is to partially disrupt fibrin polymerization to better treat coagulation-related disorders.
Acknowledgments
We would like to dedicate this manuscript to Prof. H. A. Scheraga on the occasion of his 75th birthday. We thank I. LeTrong and Dr. V. Yee for assistance with data collection and processing, and Prof. W. Hol and the Murdock Foundation for access to data collection and computational resources. This work was supported by National Institutes of Health Grants HL50355 (R.E.S.) and HL16919 (E.W.D.). H.C.F.C. was supported by a Research Fellowship from the Heart and Stroke Foundation of Canada.
Footnotes
Data deposition: The atomic coordinates and structure factors reported in this paper have been deposited in the Protein Data Bank (accession nos. 2FIB and 3FIB).
References
- 1.Davie E W, Fujikawa K, Kisiel W. Biochemistry. 1991;29:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Lorand L, Losowsky M S, Miloszewski K J M. Prog Hemost Thromb. 1980;5:245–290. [PubMed] [Google Scholar]
- 3.Hall C E, Slayter H S J. J Biophys Biochem Cytol. 1959;5:11–15. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisel J W, Stauffacher C V, Bullitt E, Cohen C. Science. 1985;230:1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- 5.Gollwitzer R, Bode W, Schramm H-J, Typke D, Guckenberger R. Ann NY Acad Sci. 1983;408:214–225. doi: 10.1111/j.1749-6632.1983.tb23246.x. [DOI] [PubMed] [Google Scholar]
- 6.Olexa S A, Budzynski A Z. Proc Natl Acad Sci USA. 1980;77:1374–1378. doi: 10.1073/pnas.77.3.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimuzu A, Nagel G M, Doolittle R F. Proc Natl Acad Sci USA. 1992;89:2888–2892. doi: 10.1073/pnas.89.7.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazumi K, Doolittle R F. Proc Natl Acad Sci USA. 1992;89:2893–2896. doi: 10.1073/pnas.89.7.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hantgan R, McDonagh J, Hermans J. Ann NY Acad Sci. 1983;408:344–366. doi: 10.1111/j.1749-6632.1983.tb23256.x. [DOI] [PubMed] [Google Scholar]
- 10.Weisel J W, Veklich Y, Gorkun O. J Mol Biol. 1993;232:285–297. doi: 10.1006/jmbi.1993.1382. [DOI] [PubMed] [Google Scholar]
- 11.Furlan M, Rupp C, Beck E A, Svendsen L V. Thromb Haemostasis. 1992;47:118–121. [PubMed] [Google Scholar]
- 12.Laudano A P, Doolittle R F. Biochemistry. 1980;19:1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- 13.Doolittle R F, Laudano A P. Protides Biol Fluids. 1980;28:311–316. [Google Scholar]
- 14.Haverkate F, Timon G. Thromb Res. 1977;10:803–812. doi: 10.1016/0049-3848(77)90137-2. [DOI] [PubMed] [Google Scholar]
- 15.Laudano A P, Cottrell B A, Doolittle R F. Ann NY Acad Sci. 1983;408:315–329. doi: 10.1111/j.1749-6632.1983.tb23254.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamazumi K, Doolittle R F. Protein Sci. 1992;1:1719–1720. doi: 10.1002/pro.5560011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieuwenhuizen W, Vermond W A, Nooijen W J, Haverkate F. FEBS Lett. 1979;98:257–259. doi: 10.1016/0014-5793(79)80194-5. [DOI] [PubMed] [Google Scholar]
- 18.Bailey O K, Astbury W T, Rudall K M. Nature (London) 1943;151:716–717. [Google Scholar]
- 19.Ferry J D. Proc Natl Acad Sci USA. 1952;38:566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheraga H A, Laskowski M., Jr Adv Protein Chem. 1957;12:1–131. [Google Scholar]
- 21.Doolittle R F. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- 22.Scheraga H A. Ann NY Acad Sci. 1983;408:330–343. doi: 10.1111/j.1749-6632.1983.tb23255.x. [DOI] [PubMed] [Google Scholar]
- 23.Shafer J A, Higgins D L. Crit Rev Clin Lab Sci. 1988;26:1–49. doi: 10.3109/10408368809105888. [DOI] [PubMed] [Google Scholar]
- 24.Blomback B. Thromb Res. 1996;83:1–75. doi: 10.1016/0049-3848(96)00111-9. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly T H, Laskowski M, Jr, Notley N, Scheraga H A. Arch Biochem Biophys. 1955;56:369–387. doi: 10.1016/0003-9861(55)90258-7. [DOI] [PubMed] [Google Scholar]
- 26.Bale M D, Muller M F, Ferry J D. Proc Natl Acad Sci USA. 1985;82:1410–1413. doi: 10.1073/pnas.82.5.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturtevant J M, Laskowski M, Jr, Donnelly T H, Scheraga H A. J Am Chem Soc. 1955;77:6168–6172. [Google Scholar]
- 28.Hudry-Clergeon G, Freyssinet J-M, Torbet J, Marx J. Ann NY Acad Sci. 1983;408:380–387. doi: 10.1111/j.1749-6632.1983.tb23258.x. [DOI] [PubMed] [Google Scholar]
- 29.Váradi A, Scheraga H A. Biochemistry. 1986;25:519–528. doi: 10.1021/bi00351a001. [DOI] [PubMed] [Google Scholar]
- 30.Dang C V, Ebert R F, Bell W R. J Biol Chem. 1985;260:9713–9719. [PubMed] [Google Scholar]
- 31.Yee V C, Pratt K P, Côté H C F, Le Trong I, Chung D W, Davie E W, Stenkamp R E, Teller D C. Structure. 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 32.Altieri D C, Plescia J, Plow E F. J Biol Chem. 1993;268:1847–1853. [PubMed] [Google Scholar]
- 33.Chen R, Doolittle R F. Biochemistry. 1971;10:4486–4491. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- 34.Farrell D H, Thiagarajan P, Chung D W, Davie E W. Proc Natl Acad Sci USA. 1992;89:10729–10732. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everse S J, Pelletier H, Doolittle R F. Protein Sci. 1995;4:1013–1016. doi: 10.1002/pro.5560040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McRee D E. J Mol Graph. 1992;10:44–46. [Google Scholar]
- 37.Brünger A T. xplor Version 3.1. A System for X-Ray Crystallography and NMR. New Haven, CT: Yale Univ. Press; 1992. [Google Scholar]
- 38.Kraulis P J. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 39.Merritt E, Murphy M. Acta Crystallogr D. 1994;50:869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 40.Nicholls A, Sharp K A, Honig B. Proteins Struct Funct Genet. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 41.Mihalyi E, Tercero J C, Diaz-Maurino T. Biochemistry. 1991;30:4753–4762. doi: 10.1021/bi00233a017. [DOI] [PubMed] [Google Scholar]
- 42.Herzberg O, Moult J. Proteins Struct Funct Genet. 1991;11:223–229. doi: 10.1002/prot.340110307. [DOI] [PubMed] [Google Scholar]
- 43.Stewart D E, Sarkar A, Wampler J E. J Mol Biol. 1990;214:253–260. doi: 10.1016/0022-2836(90)90159-J. [DOI] [PubMed] [Google Scholar]
- 44.Odefey, Mayr C M, Schmid F X. J Mol Biol. 1995;245:69–78. doi: 10.1016/s0022-2836(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 45.Mayr L M, Willbold D, Rosch P, Schmid F X. J Mol Biol. 1994;240:288–293. doi: 10.1006/jmbi.1994.1446. [DOI] [PubMed] [Google Scholar]
- 46.Dodge R W, Scheraga H A. Biochemistry. 1996;35:1548–1559. doi: 10.1021/bi952348q. [DOI] [PubMed] [Google Scholar]
- 47.Tweedy N B, Nair S K, Paterno S A, Fierke C A, Christianson D W. Biochemistry. 1993;32:10944–10949. doi: 10.1021/bi00092a003. [DOI] [PubMed] [Google Scholar]
- 48.Ebert R F, editor. Index of Variant Human Fibrinogens. Boca Raton, FL: CRC; 1994. [Google Scholar]
- 49.Okumura N, Furihata K, Terasawa F, Nakagoshi R, Ueno I, Katsuyama T. Thromb Haemostasis. 1996;75:887–891. [PubMed] [Google Scholar]
- 50.Doolittle R F, Everse S J, Spraggon G. FASEB J. 1996;10:1464–1470. doi: 10.1096/fasebj.10.13.8940292. [DOI] [PubMed] [Google Scholar]