Abstract
The relation between changes in brain and plasma concentrations of neurosteroids and the function and structure of γ-aminobutyric acid type A (GABAA) receptors in the brain during pregnancy and after delivery was investigated in rats. In contrast with plasma, where all steroids increased in parallel, the kinetics of changes in the cerebrocortical concentrations of progesterone, allopregnanolone (AP), and allotetrahydrodeoxycorticosterone (THDOC) diverged during pregnancy. Progesterone was already maximally increased between days 10 and 15, whereas AP and allotetrahydrodeoxycorticosterone peaked around day 19. The stimulatory effect of muscimol on 36Cl− uptake by cerebrocortical membrane vesicles was decreased on days 15 and 19 of pregnancy and increased 2 days after delivery. Moreover, the expression in cerebral cortex and hippocampus of the mRNA encoding for γ2L GABAA receptor subunit decreased during pregnancy and had returned to control values 2 days after delivery. Also α1,α2, α3, α4, β1, β2, β3, and γ2S mRNAs were measured and failed to change during pregnancy. Subchronic administration of finasteride, a 5α-reductase inhibitor, to pregnant rats reduced the concentrations of AP more in brain than in plasma as well as prevented the decreases in both the stimulatory effect of muscimol on 36Cl− uptake and the decrease of γ2L mRNA observed during pregnancy. These results indicate that the plasticity of GABAA receptors during pregnancy and after delivery is functionally related to fluctuations in endogenous brain concentrations of AP whose rate of synthesis/metabolism appears to differ in the brain, compared with plasma, in pregnant rats.
An important feature of γ-aminobutyric acid type A (GABAA) receptors in rat brain is their plasticity in response to exposure to brief or long-lasting physiological stimuli or to long-term pharmacological treatments. The kinetic characteristics of the various binding sites located on the GABAA receptor, as well as the density and function of these receptors in different areas of the rat brain, are affected by acute stressful stimuli (handling, foot shock, swimming), postnatal development, aging, kindling, and long-term administration of anxiolytic, hypnotic, or anticonvulsant drugs (1–6). Although recent studies have suggested that some of these changes may be mediated at the level of expression of receptor subunit genes (7–10), the chemical or molecular events that underlie such rapid, short-term, or persistent changes in the density and affinity of binding sites associated with GABAA receptors, as well as in the function of the receptor complex, remain to be characterized.
Neurosteroids accumulate in the mammalian brain in a manner that is, at least in part, independent of the peripheral tissues (adrenal glands and gonads) that synthesize these compounds (11). Systemic administration of progesterone or its metabolites 3α-hydroxy-5α-pregnan-20-one (allopregnanolone, AP) and 3α,21-dihydroxy-5α-pregnan-20-one (allotetrahydrodeoxycorticosterone, THDOC) induces anxiolytic, hypnotic, or anticonvulsant effects (for review see ref. 12) by enhancing the function of GABAA receptors (12, 13). These observations suggest that endogenous variations in the plasma and brain concentrations of these compounds induced by physiological, pharmacological, or pathological conditions might play an important role in modulation of neuronal excitability; such modulation, in turn, might result in alterations in such characteristics as emotional state, sleep pattern, and seizure threshold. Indeed, several studies have indicated that these neuroactive steroids may play a physiological role in aggression, epilepsy, and premenstrual syndrome (14–16). Consistent with these data, changes in the plasma and brain concentrations of neuroactive steroids elicited either by physiological conditions (17, 18) or by pharmacological treatments (12, 19, 20) have been associated with alterations in the behavioral and neurochemical responses linked to central GABAA receptors.
To clarify the role of the rapid effects of these neuroactive steroids on the GABAA receptor complex in the physiological modulation of receptor activity, we now have evaluated the possible functional relation between the plasticity of GABAA receptors and the changes in plasma and brain concentrations of progesterone, AP, and THDOC during pregnancy and after delivery in rats. Furthermore, we examined whether administration of finasteride, a selective blocker of 5α-reductase (21), at a dose that markedly reduces the concentrations of AP and THDOC in plasma and brain was able to reverse the changes in GABAA receptor function and structure elicited by pregnancy.
METHODS
Animals.
Adult female Sprague–Dawley rats (200–250 g; Charles River Breeding Laboratories) were studied. The animals were housed under an artificial 12-hr-light, 12-hr-dark cycle with lights on at 0800 hr.
Stage of the estrous cycle (diestrus, proestrus, or estrus) was determined from daily vaginal smears taken between 0900 and 1000 hr for 2–4 weeks. For the induction of pregnancy, females were caged with proven males on the evening of proestrus. Mating was verified by observation of spermatozoa in the vaginal smear taken the next morning, which was designated day 0 of pregnancy. Animals were killed between 0900 and 1000 hr at various stages of pregnancy or after delivery.
In one series of experiments, estrus and pregnant females were injected s.c. at the nape of the neck with finasteride (25 mg/kg) or vehicle, and killed 2, 6, or 19 hr later. In other experiments, pregnant rats were injected with the same amount of finasteride once a day (at 1400 hr) from day 12 to day 14 or day 18 of gestation. Dams were killed on day 15 or day 19 (at 0900 hr). Estrus rats were injected daily with finasteride or vehicle for 3 or 7 days. Finasteride was dissolved in a mixture of ethanol (20%, vol/vol) and corn oil (80%), and injected in a volume of 3 ml/kg; control animals received the same amount of vehicle.
Steroid Extraction and Assay.
Animals were killed either by guillotine (for plasma steroid measurements) or by focused microwave irradiation (70 W/cm2 for 4 s) to the head (for brain steroid measurements). Blood was collected from the trunk into heparinized tubes and centrifuged at 900 g for 20 min, after which the plasma was frozen until assayed for steroids.
Steroids present in cerebral cortical homogenates were extracted three times with an equal volume of ethyl acetate as previously described (22). The organic phases were applied to Seppak-silica cartridges, and steroids were further separated by HPLC on a 5-μm Lichrosorb-diol column (250 by 4 mm) (Merck) with a gradient of 2-propanol in n-hexane. The recovery of each steroid through the extraction-purification procedures (70–80%) was monitored by adding trace amounts (4,000–6,000 cpm) of 3H-labeled standards (20 to 100 Ci/mmol) to the brain tissue homogenate. Steroids were quantified by RIA as previously described (22). Plasma steroid concentrations were measured in 1 ml of plasma after extraction three times with 1.5 ml of ethyl acetate.
36Cl− Uptake.
Membrane vesicles were prepared from rat cerebral cortex as described (23). Portions (100 μl) of the vesicle preparation (1.4–1.8 mg of protein) were preincubated at 30°C for 10 min, after which 36Cl− influx was initiated by the addition of 100 μl of a solution containing 36Cl− (2 μCi/ml) in the absence or presence of muscimol. Uptake was terminated after 5 s by the addition of ice-cold buffer (3.5 ml) and rapid vacuum filtration through Whatman GF/C filters (presoaked with 0.05% polyethyleneimine to reduce nonspecific binding of 36Cl−) in a filtration manifold. The filters were washed twice with 3.5 ml of ice-cold buffer. Net uptake was calculated by subtracting 36Cl− uptake observed in the absence of muscimol (basal) from that observed in its presence. Protein concentration was measured as described (24) with BSA as standard.
Probe Preparation.
Total RNA was extracted from rat brain (25) and subjected to reverse transcription with SuperScript reverse transcriptase (Life Technologies, Gaithersburg, MD) in the presence of oligo(dT). The resulting cDNA (1–10 ng) then was subjected to PCR as previously described (25) with primers designed to amplify GABAA receptor subunit cDNA sequences that exhibit the least degree of intersubunit homology. The primers for the γ2L subunit were designed to encompass a region of DNA including the 24 bp that differ between γ2L and γ2S (nucleotides 832-1369) (26). The PCR generated two fragments (537 and 513 bp) corresponding respectively to part of the cDNA sequence of the γ2L and γ2S subunits of the GABAA receptor. The two reaction products were separated by electrophoresis on a 1.8% low melting point agarose gel, visualized by staining with ethidium bromide, excised from the gel, purified and inserted into the pAMP1 cloning vector (Life Technologies). The nucleotide sequences of the PCR products were 100% identical to those of the respective previously determined subunit DNA sequences. These two different clones were used to generate two specific probes, one selective for the γ2L and the other specific for the γ2S mRNA subunits of the GABAA receptor.
Plasmids containing the cDNA fragments corresponding to the various GABAA receptor subunits were linearized with the appropriate restriction enzymes and used as a template for transcription by RNA polymerase (SP6 or T7) to generate [α-32P]CTP-labeled cRNA probes for RNase protection assays.
RNase Protection Assay.
RNase protection assays were performed as previously described (25). Total RNA was extracted from rat cerebral cortex or hippocampus and quantified by measurement of absorbance at 260 nm, and portions of 25 μg were subjected to the assay with GABAA receptor subunit and cyclophilin cRNA probes. RNA–RNA hybrids were subjected to electrophoresis on a sequencing gel containing 5% polyacrylamide and urea and were visualized by autoradiography. The relative amounts of GABAA receptor subunit and cyclophilin (internal standard) mRNAs were evaluated by densitometry (Bio-Rad GS-700). The data were normalized by dividing the OD value of each subunit-protected fragment by that of the cyclophilin-protected fragment.
Statistical Analysis.
The statistical significance of differences was assessed by ANOVA followed by Scheffe’s test.
RESULTS
Steroid Concentrations in Plasma and Cerebral Cortex.
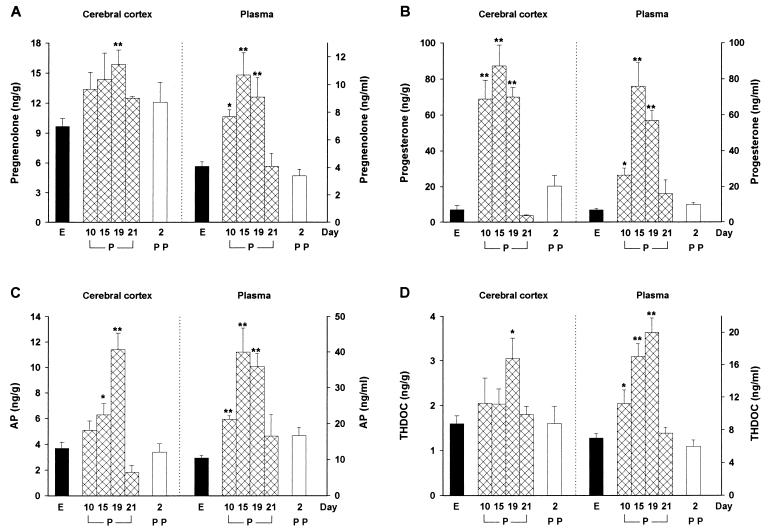
The changes in the concentrations of pregnane steroids in rat cerebral cortex during pregnancy and after delivery, compared with the corresponding concentrations on the day of estrus (control values), are shown in Fig. 1. The concentration of progesterone was increased markedly (9-fold) on day 10 of pregnancy, reached a peak (12-fold) on day 15, was still higher (10-fold) than the estrus value on day 19, and returned to control values immediately before delivery (day 21). The concentrations of pregnenolone and AP were not significantly changed on day 10 of pregnancy, showed a maximal increase (+63 and +208%, respectively) on day 19, and had returned to control values on day 21. The concentration of THDOC in the cerebral cortex remained unchanged during the first 15 days of pregnancy, was significantly increased (+90%) on day 19, and had returned to control values on day 21. The cortical concentrations of all steroids during the postpartum period did not differ from the control values.
Figure 1.
Changes in the concentrations of pregnenolone (A), progesterone (B), AP (C), and THDOC (D) in rat cerebral cortex (Left) and plasma (Right) during pregnancy and after delivery. Values are shown for estrus (E); for days 10, 15, 19, and 21 of pregnancy (P); and for 2 days postpartum (PP). Data are expressed as nanograms of steroid either per gram of cortical tissue or per milliliter of plasma, and are means ± SEM of values obtained from 8–10 rats. ∗, P < 0.05; ∗∗, P < 0.01 vs. estrus.
The time courses of changes in steroid concentrations in plasma during pregnancy and after delivery were similar to each other and somewhat different from those observed in the brain (Fig. 1). Plasma concentrations of pregnenolone, progesterone, AP, and THDOC were significantly increased (+88, +277, +102, and +60%, respectively) 10 days after copulation, were further increased (≈2.5-, 10-, 3.6-, and 2.5-fold the estrus value, respectively) on days 15–19 of pregnancy, and had returned to control values on day 21, thereafter remaining unchanged for up to 7 days after delivery (data not shown). The plasma concentration of corticosterone, the major adrenal corticosteroid in the rat, was significantly increased only on day 19 of pregnancy (estrus, 132 ± 9 ng/ml; day 19 of pregnancy, 209 ± 22 ng/ml; P < 0.05).
36Cl− Uptake.
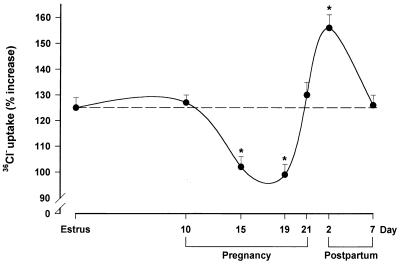
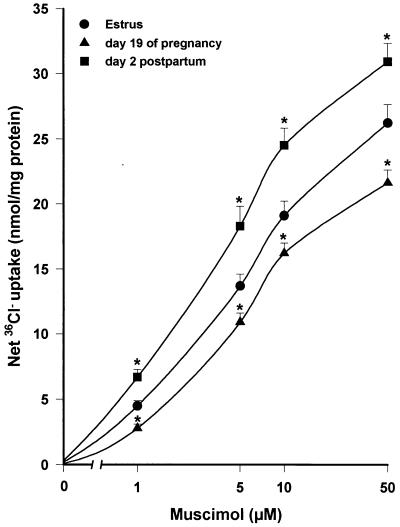
The functional state of the GABAA receptor-coupled Cl− channel during pregnancy and after delivery was investigated by measuring 36Cl− uptake by cortical membrane vesicles. As expected (23), muscimol (5 μM) stimulated (+125%) 36Cl− uptake by membrane vesicles prepared from female rats killed during estrus (Fig. 2). The stimulatory effect of muscimol remained unchanged on day 10 of pregnancy, but was reduced on day 15 (−21%) and on day 19 (−24%) of pregnancy compared with the estrus value. In contrast, the efficacy of muscimol in stimulating 36Cl− uptake was increased (+32%) 2 days after delivery, before returning to control values by 7 days after delivery. A similar pattern of stimulation also was observed in the presence of lower (1 μM) or higher (10 and 50 μM) concentrations of muscimol (Fig. 3).
Figure 2.
Effects of muscimol on 36Cl− uptake by membrane vesicles prepared from the cerebral cortex of rats during pregnancy and after delivery. Membrane vesicles, prepared from rats on the indicated days, were incubated for 10 min at 30°C before the addition of 36Cl− in the absence (basal value) or presence of 5 μM of muscimol. Uptake of 36Cl− was terminated 5 s later. Data are expressed as the percentage increase in 36Cl− uptake induced by 5 μM of muscimol relative to the basal value and are means ± SEM of six different experiments, each performed with two rats per group. ∗, P < 0.05 vs. estrus.
Figure 3.
Effects of muscimol concentration on 36Cl− uptake by membrane vesicles prepared from the cerebral cortex of rats in estrus, on day 19 of pregnancy, or 2 days after delivery. Net uptake indicates the influx of 36Cl− (nmol/mg of protein) in 5 s induced by muscimol at 1–50 μM (basal uptake was subtracted from all values). Data are means ± SEM from 4–7 different experiments, each performed with two rats per group. Basal 36Cl− uptake values (nmol/mg of protein) were 11.2 ± 0.4 (estrus), 10.8 ± 0.5 (day 19 of pregnancy), and 11.7 ± 0.6 (2 days after delivery). ∗, P < 0.05 vs. corresponding estrus value.
Expression Levels of GABAA Receptor γ2L Subunit mRNA.
The GABAA receptor subunit mRNA expression in the cerebral cortex and hippocampus of rats in estrus, during pregnancy (day 19), and after delivery (day 2) was measured by RNase protection assay. The expression level of transcripts encoding for the γ2L subunit in both the cerebral cortex and hippocampus was decreased by ≈25% on day 19 of pregnancy, but had returned to control values by the second day after delivery (Table 1). No significant changes were apparent for the expression of mRNAs encoding the α1, α2, α3, α4, β1, β2, β3, or γ2S subunits of the GABAA receptor in either the cerebral cortex or hippocampus (data not shown).
Table 1.
Effects of pregnancy and delivery on the amount of GABAA receptor γ2L subunit mRNA in the cerebral cortex and hippocampus
| γ2L mRNA, % estrus
|
||
|---|---|---|
| Cerebral cortex | Hippocampus | |
| Estrus | 100 ± 1 | 100 ± 2 |
| Day 19 of pregnancy | 75 ± 3a | 77 ± 3a |
| Day 2 after delivery | 98 ± 6 | 112 ± 5 |
Data are expressed as a percentage of the values for control rats in estrus and are means ± SEM of 30 animals (10 experiments) for each experimental group.
P < 0.001 vs. estrus.
Effects of Finasteride on Neurosteroid and GABAA Receptor Expression.
To clarify the roles of AP and THDOC in the changes in GABAA receptor structure and function during pregnancy, we investigated the effects of finasteride administration (a specific 5α-reductase inhibitor; ref. 21), on both muscimol-stimulated 36Cl− uptake and the amount of γ2L subunit mRNA. A single administration of this drug (25 mg/kg, s.c.) reduced the concentrations of AP and THDOC both in estrus rats (≈−65% and −60% in the cerebral cortex and plasma, respectively) and in rats on day 19 of pregnancy (≈−80% and −55% in the cerebral cortex and plasma, respectively) (Table 2). The finasteride-induced inhibition of AP and THDOC production lasted longer than 19 hr, although at this time the brain AP and THDOC concentrations had begun to return to pretreatment values.
Table 2.
Effects of acute administration of finasteride on cerebrocortical and plasma concentrations of progesterone, AP, and THDOC of estrus and pregnant rats
| Cerebral cortex, ng/g
|
Plasma, ng/ml
|
|||||
|---|---|---|---|---|---|---|
| Progesterone | AP | THDOC | Progesterone | AP | THDOC | |
| Estrus | 7.9 ± 1.4 | 3.0 ± 0.3 | 1.5 ± 0.2 | 6.9 ± 1.8 | 15 ± 1.8 | 7.8 ± 1.0 |
| (+)Finasteride (2 hr) | 18.3 ± 3.0 | 0.7 ± 0.08b | 0.4 ± 0.07b | 13.0 ± 3.6 | 5.2 ± 1.3a | 2.9 ± 0.7a |
| (+)Finasteride (6 hr) | 11.0 ± 2.1 | 0.8 ± 0.1b | 0.5 ± 0.09b | 7.9 ± 1.3 | 5.4 ± 1.2a | 3.1 ± 0.5a |
| (+)Finasteride (19 hr) | 7.5 ± 0.9 | 1.4 ± 0.09b | 0.7 ± 0.07a | 7.7 ± 1.6 | 7.6 ± 1.0a | 4.0 ± 0.6a |
| Pregnant | 104 ± 6.6 | 12.2 ± 1.6 | 2.8 ± 0.4 | 45 ± 5.8 | 55 ± 6.7 | 26 ± 2.5 |
| (+)Finasteride (2 hr) | 144 ± 7.5c | 1.2 ± 0.1d | 0.3 ± 0.09d | 51 ± 8.5 | 29 ± 3.2c | 10 ± 1.6c |
| (+)Finasteride (6 hr) | 161 ± 10.3c | 1.3 ± 0.2d | 0.5 ± 0.1d | 60 ± 10 | 23 ± 2.6c | 11 ± 1.8c |
| (+)Finasteride (19 hr) | 102 ± 7.4 | 3.5 ± 0.6d | 1.0 ± 0.1c | 58 ± 7.8 | 26 ± 2.8c | 13 ± 1.8c |
Rats in estrus or on day 19 of pregnancy were injected with finasteride (25 mg/kg, s.c.) or vehicle at the indicated times before killing. Data are means ± SEM of values obtained from 10 rats.
P < 0.05,
P < 0.01 vs. the corresponding estrus value;
P < 0.05,
P < 0.01 vs. the corresponding pregnant value.
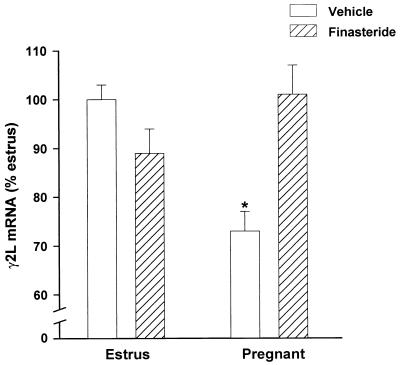
To maximally inhibit the pregnancy-associated increases in AP and THDOC, dams were treated daily with finasteride from day 12 to day 18 of pregnancy and killed on day 19 (19 hr after the last injection). Such repeated daily treatment markedly reduced the pregnancy-induced increases in the amounts of AP and THDOC, being the inhibition of AP production greater in cortex than in plasma whereas that of THDOC was similar in the two tissues (Table 3). This finasteride treatment abolished the decrease in the stimulatory effect of muscimol (1–50 μM) on 36Cl− uptake normally apparent on day 19 of pregnancy (Table 4). Also such finasteride treatment prevented the pregnancy-induced decrease in the expression of γ2L subunit mRNA in the hippocampus (Fig. 4). A similar finasteride treatment in estrus females reduced brain concentrations by an extent smaller than in cortex of pregnant dams (Table 3) and failed to modify muscimol-stimulated 36Cl− uptake (Table 4) or the amount of γ2L subunit mRNA in the hippocampus (Fig. 4). Finally, finasteride given from days 12 to 14 of pregnancy also prevented the decrease in the ability of muscimol (1 μM) to stimulate 36Cl− uptake by cortical membrane vesicles measured on day 15 of pregnancy (net 36Cl− uptake: estrus 3.8 ± 0.2 nmol/mg of protein; pregnant 2.6 ± 0.2 nmol/mg of protein; pregnant + finasteride 4.0 ± 0.4 nmol/mg of protein).
Table 3.
Effects of subchronic treatment with finasteride on cerebrocortical and plasma concentrations of progesterone, AP, and THDOC in estrus and pregnant rats
| Cerebral cortex, ng/g
|
Plasma, ng/ml
|
|||||
|---|---|---|---|---|---|---|
| Progesterone | AP | THDOC | Progesterone | AP | THDOC | |
| Estrus | 7.1 ± 2.5 | 3.7 ± 0.5 | 1.3 ± 0.1 | 6.8 ± 0.8 | 13.3 ± 1.2 | 8.8 ± 0.7 |
| Estrus + finasteride | 6.8 ± 3.1 | 2.0 ± 0.3a | 0.7 ± 0.1a | 7.3 ± 1.0 | 7.8 ± 1.1a | 5.1 ± 0.8a |
| Pregnant | 70.0 ± 8.5 | 15.9 ± 2.5 | 2.6 ± 0.4 | 58.0 ± 8.0 | 39.0 ± 3.0 | 24.9 ± 2.7 |
| Pregnant + finasteride | 68.6 ± 7.6 | 5.8 ± 1.4c | 1.0 ± 0.3b | 55.2 ± 8.5 | 22.4 ± 2.5b | 14.2 ± 1.6c |
Finasteride (25 mg/kg, s.c.) or vehicle was injected daily from day 12 to day 18 of pregnancy, and rats were killed on day 19. Estrus rats were similarly treated for 7 days with finasteride. Data are means ± SEM of values obtained from 15 rats.
P < 0.05 vs. the corresponding estrus value;
P < 0.05,
P < 0.01 vs. the corresponding pregnant value.
Table 4.
Effects of subchronic treatment with finasteride on muscimol-stimulated 36Cl− uptake by cerebrocortical membrane vesicles of estrus and pregnant rats
| Net 36Cl− uptake, nmol/mg protein
|
||||
|---|---|---|---|---|
| Estrus | Estrus + finasteride | Pregnant | Pregnant + finasteride | |
| Muscimol, 1 μM | 4.1 ± 0.3 | 4.6 ± 0.4 | 2.7 ± 0.3a | 4.9 ± 0.4 |
| Muscimol, 5 μM | 15.2 ± 0.7 | 16.9 ± 0.8 | 12.4 ± 0.6a | 16.2 ± 0.6 |
| Muscimol, 50 μM | 25.9 ± 1.6 | 28.1 ± 2.1 | 19.9 ± 1.2a | 27.7 ± 1.9 |
Finasteride (25 mg/kg, s.c.) or vehicle was injected daily from day 12 to day 18 of pregnancy, and rats were killed on day 19. Estrus rats were similarly treated with finasteride for 7 days. Net uptake indicates the influx of 36Cl− (nmol/mg of protein) in 5 s induced by muscimol (basal uptake was subtracted from all values). Data are means ± SEM from five different experiments, each performed with two rats per group.
P < 0.05 vs. corresponding estrus value.
Figure 4.
Effects of subchronic treatment with finasteride on γ2L subunit mRNA expression in the hippocampus of estrus and pregnant rats. Finasteride (25 mg/kg, s.c.) or vehicle was injected daily from day 12 to day 18 of pregnancy, and rats were killed on day 19. Estrus rats were similarly treated with finasteride for 7 days. Data are expressed as a percentage of the estrus value and are means ± SEM of 12–14 animals for each group. ∗, P < 0.01 vs. estrus.
DISCUSSION
We have shown that the changes in GABAA receptor responsiveness to muscimol and subunit mRNA expression that occur in rat brain during pregnancy and after delivery are related to fluctuations of the brain cortical concentrations of AP. Whereas the kinetics of changes in the concentrations of progesterone, AP, and THDOC were similar in plasma, they markedly differed in the cerebral cortex where the surge of AP was slower than that of progesterone and reached a peak on day 19 of pregnancy, at the time when the GABAA receptor responsiveness and the γ2L subunit expression were maximally decreased. In contrast, brain progesterone concentration followed its plasma levels, being maximally increased between days 12 and 15 and remaining at a plateau up to day 19. A similar dissociation between progesterone and AP concentrations also has been detected in human cerebrospinal fluid (27).
The present results suggest that in the brain of pregnant rats the synthesis/accumulation of AP is not simply a function of the plasma progesterone concentration. Moreover, the greater decline of AP concentration induced by finasteride in the brain of pregnant dams, compared with their plasma and to the brain of estrus rats (Table 2), invites us to speculate that the rate of synthesis of AP in the brain cortex is greater than in plasma in pregnant rats, and increased in the latter compared with estrus rats. Henceforth, it might inferred that by inhibiting the AP synthesis and observing its decline at short times after finasteride administration, one may be enable to measure the AP turnover rate, as a better functional index than the changes in concentrations. One possible explanation for the different rates of AP decline in pregnant and estrus rats is that steroid hormones or other agents may regulate the activity or expression of either 5α-reductase or 3α-hydroxysteroid oxidoreductase in brain during pregnancy therefore selectively changing rates of AP synthesis. Indeed, estradiol has been shown to regulate 3α-hydroxysteroid oxidoreductase activity in rat brain (28). Thus, one may suggest a dynamic regulation of steady state: for instance, a high plasma level of progesterone may be more economically maintained with an inhibition of progesterone metabolism by progesterone. Such a mechanism can be studied by measuring turnover rates at high or low steady state.
Measurements of 36Cl− uptake by cerebrocortical membrane vesicles revealed a progressive decrease in the stimulatory effect of muscimol that peaked during the last third of pregnancy, with a sudden increase immediately before delivery and a further increase 2 days after delivery. Because the tone of GABAergic transmission is a function of GABA turnover rate and release, and denervation is characterized by receptor supersensitivity, it might be inferred that the aforementioned changes in GABAA receptor responsiveness to muscimol reflect to some extent a decreased and an increased GABAergic inhibitory activity during pregnancy and after delivery, respectively.
The changes in GABAA receptor sensitivity to muscimol during pregnancy and after delivery were associated with changes in the expression of γ2L subunit mRNA that was decreased in the cerebral cortex and hippocampus during pregnancy and returned to control values 2 days after delivery. The observation that the amounts of α1, α2, α3, α4, β1, β2, β3, and γ2S subunit mRNAs were not significantly affected by pregnancy or delivery suggests that the changes in γ2L subunit mRNA expression are specific. However, it will be interesting to evaluate whether the γ1 subunit expression was changed, because the presence of this subunit is associated with an increased efficacy of neurosteroids on GABAA receptors (29). The γ2 subunit has been shown to be essential for the modulatory action of benzodiazepines (30), and the probability of Cl− channel opening during agonist occupation of the receptor is limited when this subunit is not present (31). Thus, it is possible that changes in the expression of genes encoding γ1 and γ2 receptor subunits may contribute to the changes in the efficacy of muscimol in enhancing 36Cl− uptake during pregnancy and after delivery.
Several studies have indicated that “in vivo” or “in vitro” long-term treatment with neuroactive steroids decrease the efficacy of GABA and its allosteric modulators on GABAA receptors (25, 32, 33). Therefore, our data suggest that the increase in brain AP concentrations during the last third of pregnancy can be compared with a long-term administration of high doses of this neuroactive steroid, whereas the marked fall in its concentration at the end of pregnancy and after delivery is similar to a sudden discontinuation of such treatment. As recently shown (34), the induction of progesterone withdrawal results in a decrease in the total hippocampal GABAA receptor-mediated current associated with an increased expression of α4 GABAA receptor subunit in the hippocampus. We did not observe this latter change in our experimental model. However, the reason for this apparent discrepancy may be related to the substantial difference in the experimental systems because the complex hormonal changes associated with pregnancy cannot be replicated by the administration of a single hormone. Nevertheless, both the pharmacological data (34) and our physiological study indicate that the plasticity of GABAA receptors is functionally related to fluctuations in endogenous steroid concentrations.
This latter conclusion is supported by the finding that a treatment with finasteride, an inhibitor of 5α-reductase, the enzyme that converts progesterone to the AP precursor 5α-dihydroprogesterone from days 12 to 18 of pregnancy prevented the AP surge in brain cortex as well as abolished the decreases in both the sensitivity of GABAA receptors to the action of muscimol and the expression of γ2L subunit mRNA normally observed during pregnancy. Moreover, a shorter treatment (from days 12 to 14) with finasteride induced a similar effect on GABAA receptor responsiveness measured on day 15 of pregnancy. The greater decline in AP brain concentrations, compared with plasma, elicited by finasteride treatment in pregnant dams was unexpected, given the reported higher affinity of this compound for type 2 (peripheral steroidogenic tissues) than for type 1 (brain) 5α-reductase (21). Although 5α-reductase type 2 has not been detected in adult rat brain tissue ex vivo, the observation that the expression of this isozyme is transiently and androgen-dependently increased (male > female) during embryonic development (35) suggests that one should test whether the marked increases in the concentrations of certain steroid hormones during pregnancy might affect the expression of 5α-reductase type 2 in brain. However, because the doses of finasteride used by us were elevated and repeated for several days, the susceptibility of 5α-reductase type 1 to inhibition by finasteride also might have been reached in our experiments.
It remains to be determined whether the changes in GABAA receptor function and subunit expression during pregnancy are because of direct or indirect (genomic) actions of neurosteroids. The oxidation of AP and THDOC, which may occur in vivo, yields 5α-dihydroprogesterone (5α-DHP) and 5α-dihydrodeoxycorticosterone (5α-DHDOC), both of which bind to and activate the cytosolic progesterone receptor (36). However, a GABAA receptor subunit expression regulated by progesterone receptors occupied by 5α-DHP and 5α-DHDOC appears unlikely. Indeed, the high brain concentration of progesterone during pregnancy, which are finasteride resistant, would be expected to preclude an action of 5α-DHP or 5α-DHDOC at progesterone receptors. This conclusion cannot exclude a genomic action of progesterone on other transcription regulatory proteins operative in the regulation of transcription or translation of mRNAs encoding for GABAA receptor subunits during pregnancy. However, the finasteride-induced reversal of the increases in AP brain concentrations and of the changes in GABAA receptor responsiveness to the agonist and γ2L subunit gene expression that occur during pregnancy suggest that these latter changes are the consequence of a AP action at the steroid recognition site on the GABAA receptor. Accordingly, previous studies have shown that progesterone metabolites may modulate GABAA receptor subunit gene expression by a similar mechanism (37).
In conclusion, our data demonstrate a functional relation between the fluctuations in the cerebrocortical concentrations of neurosteroids and GABAA receptor plasticity during pregnancy and after delivery.
Acknowledgments
This research was supported by Grant 97.06152855 from Ministero dell’Universitá e della Ricerca Scientifica e Technologica (Projects of National Relevance, Article 65 DPR 382/80).
ABBREVIATIONS
- GABAA
γ-aminobutyric acid type A
- AP
allopregnanolone
- THDOC
allotetrahydrodeoxycorticosterone
References
- 1. Biggio G, Corda M G, Concas A, Demontis G, Rossetti Z, Gessa G L. Brain Res. 1981;229:363–369. doi: 10.1016/0006-8993(81)91000-3. [DOI] [PubMed] [Google Scholar]
- 2.Havoundjian H, Paul S M, Skolnick P. J Pharmacol Exp Ther. 1986;237:787–793. [PubMed] [Google Scholar]
- 3.Eichinger A, Sieghart W. J Neurochem. 1986;46:173–180. doi: 10.1111/j.1471-4159.1986.tb12941.x. [DOI] [PubMed] [Google Scholar]
- 4.Concas A, Pepitoni S, Atsoggiu T, Biggio G. Life Sci. 1988;43:1761–1771. doi: 10.1016/0024-3205(88)90275-5. [DOI] [PubMed] [Google Scholar]
- 5.Titulaer M N G, Kamphuis W, Lopes da Silva F H. Neuroscience. 1995;68:399–406. doi: 10.1016/0306-4522(95)00158-f. [DOI] [PubMed] [Google Scholar]
- 6.Miller L G, Greenblatt D J, Barnhill J G, Shader R I. J Pharmacol Exp Ther. 1988;246:170–176. [PubMed] [Google Scholar]
- 7.Laurie D J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez A, Khan Z U, Miralles C P, Mehta A K, Ruano D, Araujo F, Vitorica J, De Blas A L. Mol Brain Res. 1997;45:59–70. doi: 10.1016/s0169-328x(96)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Rosemberg H C, Chiu T H, Zhao T-J. J Mol Neurosci. 1994;5:105–120. doi: 10.1007/BF02736752. [DOI] [PubMed] [Google Scholar]
- 10.Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy Y M, Costa E, Guidotti A. Mol Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- 11.Hu Z Y, Bourreau E, Jung-Testas I, Robel P, Baulieu E E. Proc Natl Acad Sci USA. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majewska M D. Progr Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 13.Puia G, Santi M R, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 14.Fraile I, McEwen B S, Pfaff D W. Physiol Behav. 1987;39:225–229. doi: 10.1016/0031-9384(87)90013-8. [DOI] [PubMed] [Google Scholar]
- 15.Herzog A G. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Seippel L, Purdy R H, Backstrom T. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 17.Finn D A, Gee K W. J Pharmacol Exp Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- 18.Halbreich U, Petty F, Yonkers K, Kramer G L, Rush J, Bibi K W. Am J Psychiatry. 1996;153:718–720. doi: 10.1176/ajp.153.5.718. [DOI] [PubMed] [Google Scholar]
- 19.McAuley J W, Reynolds I J, Kroboth F J, Smith R B, Kroboth P D. J Clin Psychopharmacol. 1995;15:3–11. doi: 10.1097/00004714-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Freeman E W, Purdy R H, Contifaris C, Rickels K, Paul S M. Clin Neuroendocrinol. 1993;58:478–484. doi: 10.1159/000126579. [DOI] [PubMed] [Google Scholar]
- 21.Azzolina B, Ellsworth K, Andersson S, Geissler W, Bull H G, Harris G S. J Steroid Biochem Mol Biol. 1997;61:55–64. doi: 10.1016/s0960-0760(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Barbaccia M L, Roscetti G, Trabucchi M, Mostallino M C, Concas A, Purdy R H, Biggio G. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- 23.Harris R A, Allan A M. Science. 1985;228:1108–1110. doi: 10.1126/science.2581319. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Yu R, Follesa P, Ticku M K. Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- 26.Whiting P, McKernan R M, Iversen L L. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uzunova V, Sheline Y, Davis J M, Rasmusson A, Uzunov D, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1998;45:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penning T M, Sharp R B, Krieger N R. J Biol Chem. 1985;260:15266–15272. [PubMed] [Google Scholar]
- 29.Puia G, Ducic I, Vicini S, Costa E. Recept Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 30.Pritchett D B, Sontheimer H, Shivers B D, Ymer S, Kettermann H, Schofield P R, Seeburg P H. Nature (London) 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 31.Horne A L, Harkness P C, Hadingham K L, Whiting P, Kemp J A. Br J Pharmacol. 1993;108:711–716. doi: 10.1111/j.1476-5381.1993.tb12866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu R, Ticku M K. Mol Pharmacol. 1995;47:603–610. [PubMed] [Google Scholar]
- 33.Costa A M, Spence K T, Smith S S, ffrench-Mullen J M. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- 34.Smith S S, Gong Q H, Hsu F, Markowitz R S, ffrench-Mullen J M H, Li X. Nature (London) 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 35.Poletti A, Negri-Cesi P, Rabuffetti M, Colciago A, Celotti F, Martini L. Endocrinology. 1998;139:2171–2178. doi: 10.1210/endo.139.4.5866. [DOI] [PubMed] [Google Scholar]
- 36.Rupprecht R, Reul J M H M, Trapp T, van Steense B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- 37.Weiland N G, Orchinik M. Mol Brain Res. 1995;32:271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]