Abstract
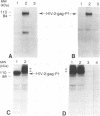
It has been difficult to evaluate the role of individual viral proteins in poliovirus replication because a suitable complementation system has not yet been developed. To approach this problem, we constructed a chimeric human immunodeficiency virus type 2 (HIV-2)-gag-poliovirus minireplicon in which regions of the gag gene of HIV-2 were inserted in the poliovirus genome between nucleotides 1174 and 2470. Transfection of this chimeric RNA into HeLa cells results in the replication of the minireplicon and expression of an HIV-2-gag-P1 fusion protein which can be immunoprecipitated with antibodies to HIV-2-gag. Expression of the HIV-2-gag-P1 fusion protein was dependent on replication of the chimeric RNA genome. Although the chimeric HIV-2-gag-poliovirus RNA genome replicated in poliovirus-infected cells, transfection of the chimeric HIV-2-gag-poliovirus genome into vaccinia virus-infected cells resulted in increased replication as measured by analysis of chimeric RNA. The increase in replication correlated with an increase in the expression of the HIV-2-gag-P1 fusion protein in vaccinia virus-infected cells. To characterize this system, we constructed a mutation in the 2A gene to change a cysteine at amino acid 109 to a serine. Expression of the HIV-2-gag-P1 fusion protein was not detected when the HIV-2-gag-poliovirus genome containing the 2A mutation was transfected into HeLa cells, demonstrating the mutation was lethal for replication. When the chimeric genome was transfected into poliovirus-infected cells, no RNA replication or expression of the HIV-2-gag-P1 fusion protein was observed. In contrast, transfection of this genome into vaccinia virus-infected cells resulted in replication of the chimeric RNA and expression of two proteins with larger molecular masses than the HIV-2-gag-P1 proteins, possibly representing HIV-2-gag-P1-2A and HIV-2-gag-P1-2ABC fusion proteins. The transfection of the chimeric HIV-2-gag-poliovirus genome containing the 2A mutation into poliovirus-vaccinia virus coinfected cells resulted in the expression and partial processing of the two larger HIV-2-gag-P1 fusion proteins to give the correct molecular mass for the HIV-2-gag-P1 fusion protein. The 2A mutation was reconstructed back into the full-length infectious cDNA of poliovirus. Transfection of this cDNA into vaccinia virus-infected cells followed by immunoprecipitation with anticapsid antibodies demonstrated the presence of two proteins with molecular masses larger than P1, possibly P1-2A and P1-2ABC fusion proteins.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansardi D. C., Porter D. C., Morrow C. D. Coinfection with recombinant vaccinia viruses expressing poliovirus P1 and P3 proteins results in polyprotein processing and formation of empty capsid structures. J Virol. 1991 Apr;65(4):2088–2092. doi: 10.1128/jvi.65.4.2088-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K., Merrick W. C. Mechanism of selective translation of vaccinia virus mRNAs: differential role of poly(A) and initiation factors in the translation of viral and cellular mRNAs. J Virol. 1991 Aug;65(8):4449–4460. doi: 10.1128/jvi.65.8.4449-4460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Sarnow P., Baltimore D. Genetic complementation among poliovirus mutants derived from an infectious cDNA clone. J Virol. 1986 Dec;60(3):1040–1049. doi: 10.1128/jvi.60.3.1040-1049.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. D., Sonenberg N., Baltimore D. Poliovirus mutant that does not selectively inhibit host cell protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2913–2923. doi: 10.1128/mcb.5.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black T. L., Safer B., Hovanessian A., Katze M. G. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J Virol. 1989 May;63(5):2244–2251. doi: 10.1128/jvi.63.5.2244-2251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charini W. A., Burns C. C., Ehrenfeld E., Semler B. L. trans rescue of a mutant poliovirus RNA polymerase function. J Virol. 1991 May;65(5):2655–2665. doi: 10.1128/jvi.65.5.2655-2665.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. S., Pal-Ghosh R., Morrow C. D. Expression of human immunodeficiency virus type 1 (HIV-1) gag, pol, and env proteins from chimeric HIV-1-poliovirus minireplicons. J Virol. 1991 Jun;65(6):2875–2883. doi: 10.1128/jvi.65.6.2875-2883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis P. S., O'Donnell B. J., Barton D. J., Rogers J. A., Flanegan J. B. Replication of poliovirus RNA and subgenomic RNA transcripts in transfected cells. J Virol. 1992 Nov;66(11):6480–6488. doi: 10.1128/jvi.66.11.6480-6488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E. Poliovirus-induced inhibition of host-cell protein synthesis. Cell. 1982 Mar;28(3):435–436. doi: 10.1016/0092-8674(82)90195-7. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino-Yamagishi K., Nomoto A. In vitro construction of poliovirus defective interfering particles. J Virol. 1989 Dec;63(12):5386–5392. doi: 10.1128/jvi.63.12.5386-5392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Fäcke M., Kräusslich H. G., Lee C. K., Wimmer E. Characterization of poliovirus 2A proteinase by mutational analysis: residues required for autocatalytic activity are essential for induction of cleavage of eukaryotic initiation factor 4F polypeptide p220. J Virol. 1991 Aug;65(8):4226–4231. doi: 10.1128/jvi.65.8.4226-4231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore J., De Geus B., Jackson R. J., Pouwels P. H., Enger-Valk B. E. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1 in vitro. J Gen Virol. 1988 Jul;69(Pt 7):1627–1636. doi: 10.1099/0022-1317-69-7-1627. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Racaniello V. R. Construction and characterization of poliovirus subgenomic replicons. J Virol. 1988 May;62(5):1687–1696. doi: 10.1128/jvi.62.5.1687-1696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Hui H. X., Kappes J. C., Haggarty B. S., Hoxie J. A., Arya S. K., Shaw G. M., Hahn B. H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990 Feb;64(2):890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Alternate poliovirus nonstructural protein processing cascades generated by primary sites of 3C proteinase cleavage. Virology. 1992 Nov;191(1):309–320. doi: 10.1016/0042-6822(92)90193-s. [DOI] [PubMed] [Google Scholar]
- Lloyd R. E., Grubman M. J., Ehrenfeld E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988 Nov;62(11):4216–4223. doi: 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Gibbons G. F., Dasgupta A. The host protein required for in vitro replication of poliovirus is a protein kinase that phosphorylates eukaryotic initiation factor-2. Cell. 1985 Apr;40(4):913–921. doi: 10.1016/0092-8674(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. E., Racaniello V. R. Inhibition of translation in cells infected with a poliovirus 2Apro mutant correlates with phosphorylation of the alpha subunit of eucaryotic initiation factor 2. J Virol. 1989 Dec;63(12):5069–5075. doi: 10.1128/jvi.63.12.5069-5075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Dorner A. J., Wimmer E. Production of infectious poliovirus from cloned cDNA is dramatically increased by SV40 transcription and replication signals. Nucleic Acids Res. 1984 Jun 25;12(12):5123–5141. doi: 10.1093/nar/12.12.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Trono D., Pelletier J., Sonenberg N., Baltimore D. Translation in mammalian cells of a gene linked to the poliovirus 5' noncoding region. Science. 1988 Jul 22;241(4864):445–448. doi: 10.1126/science.2839901. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. Processing determinants required for in vitro cleavage of the poliovirus P1 precursor to capsid proteins. J Virol. 1987 Oct;61(10):3181–3189. doi: 10.1128/jvi.61.10.3181-3189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., Lloyd R. E. Characterization of the roles of conserved cysteine and histidine residues in poliovirus 2A protease. Virology. 1992 Feb;186(2):725–735. doi: 10.1016/0042-6822(92)90039-r. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Lloyd R. E. Identification of essential amino acid residues in the functional activity of poliovirus 2A protease. Virology. 1991 Jun;182(2):615–625. doi: 10.1016/0042-6822(91)90602-8. [DOI] [PubMed] [Google Scholar]