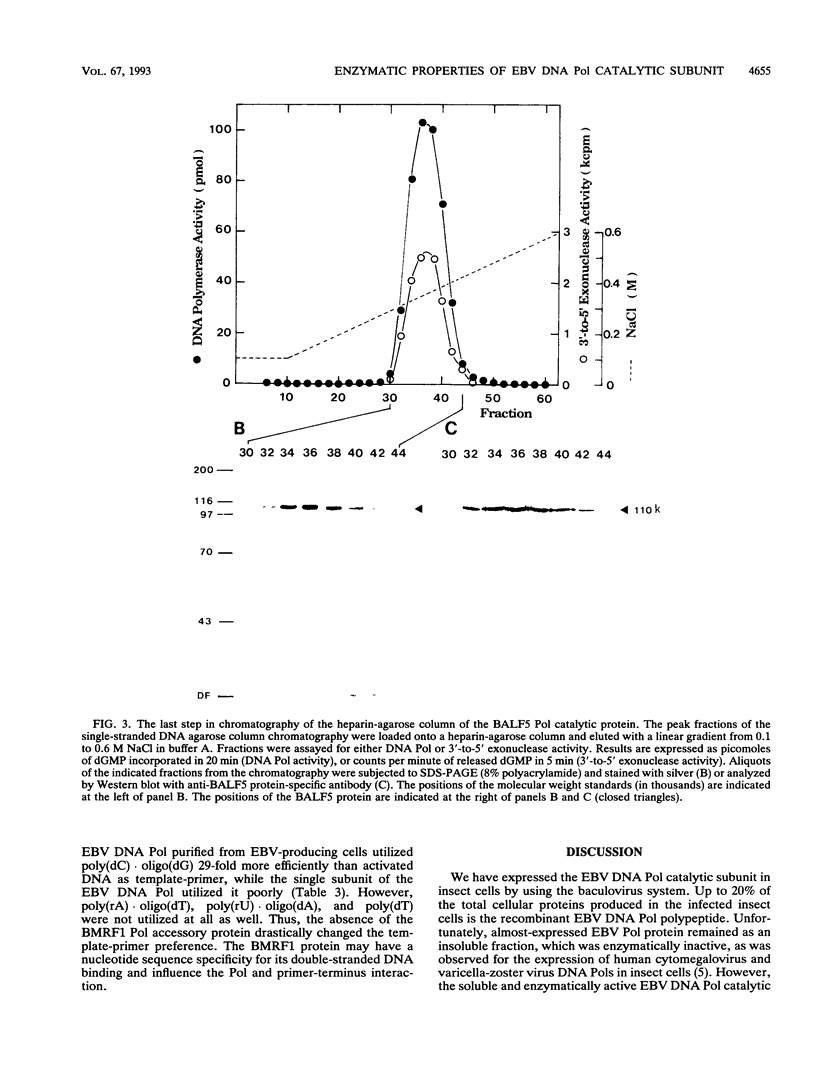
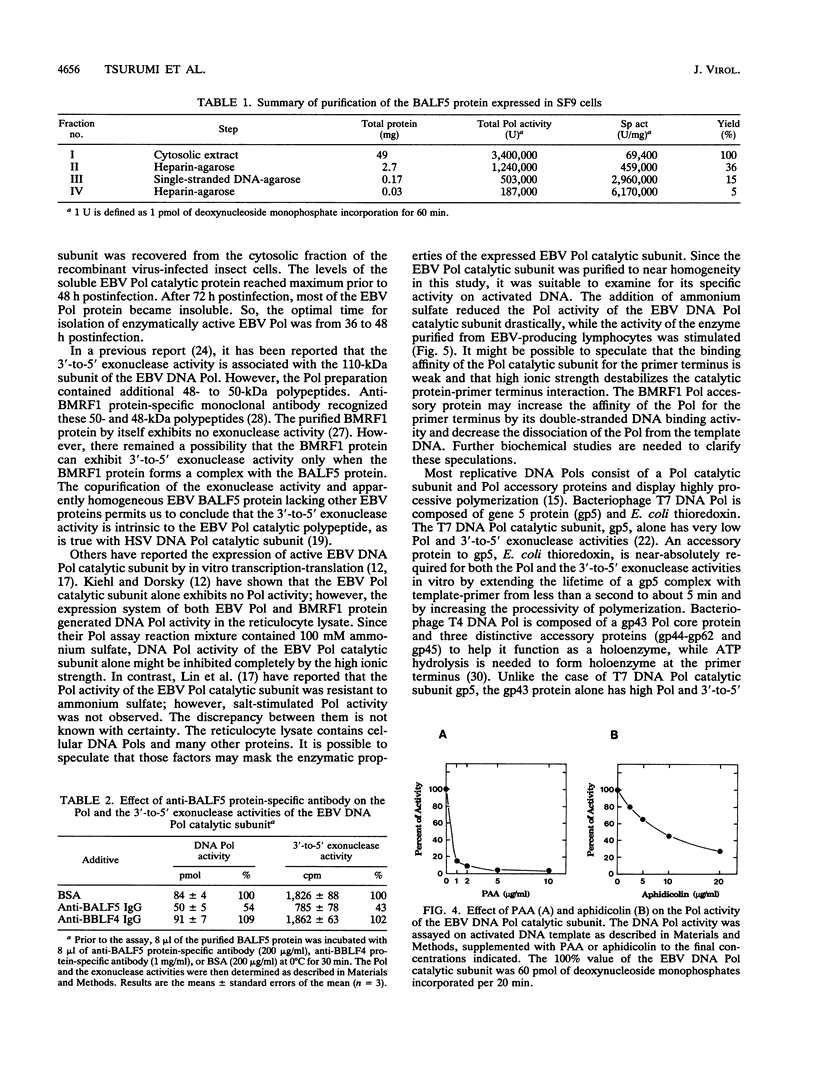
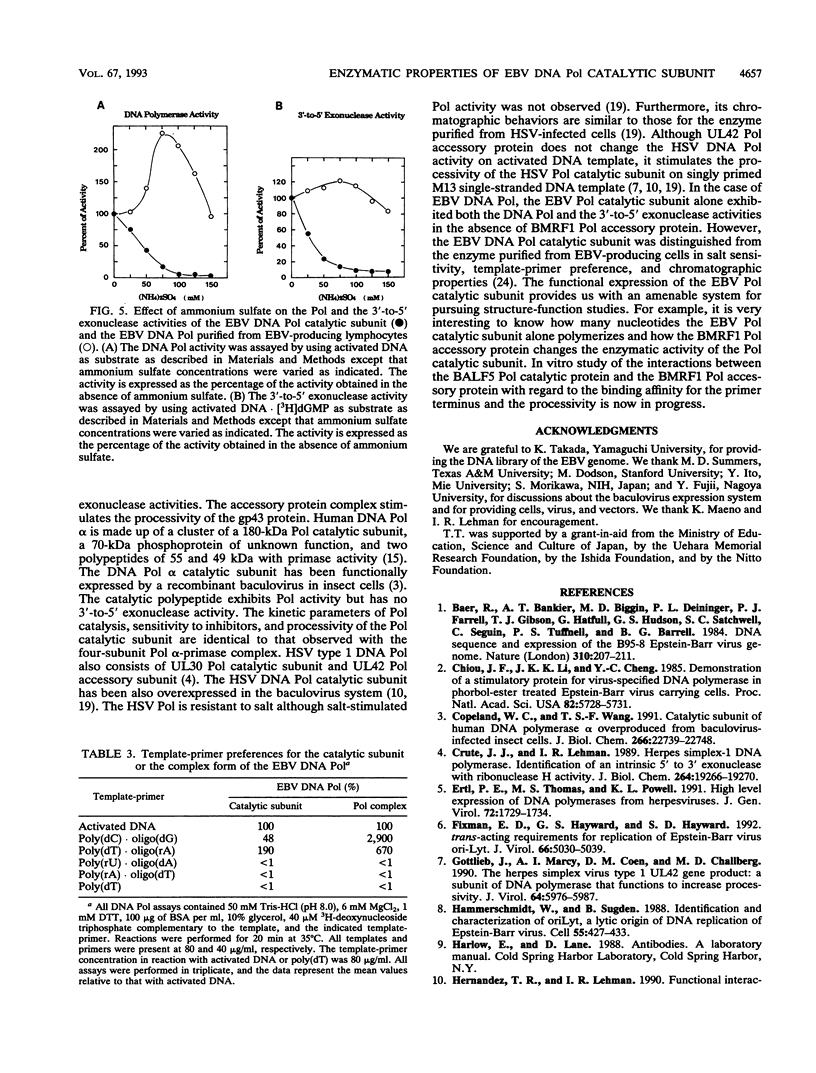
Abstract
A recombinant baculovirus containing the complete sequence for the Epstein-Barr virus (EBV) DNA polymerase catalytic subunit, BALF5 gene product, under the control of the baculovirus polyhedrin promoter was constructed. Insect cells infected with the recombinant virus produced a protein of 110 kDa, recognized by anti-BALF5 protein-specific polyclonal antibody. The expressed EBV DNA polymerase catalytic polypeptide was purified from the cytosolic fraction of the recombinant virus-infected insect cells. The purified protein exhibited both DNA polymerase and 3'-to-5' exonuclease activities, which were neutralized by the anti-BALF5 protein-specific antibody. These results indicate that the 3'-to-5' exonuclease activity associated with the EBV DNA polymerase (T. Tsurumi, Virology 182:376-381, 1991) is an inherent feature of the polymerase catalytic polypeptide. The DNA polymerase and the exonuclease activities of the EBV DNA polymerase catalytic subunit were sensitive to ammonium sulfate in contrast to those of the polymerase complex purified from EBV-producing lymphoblastoid cells, which were stimulated by salt. Furthermore, the template-primer preference for the polymerase catalytic subunit was different from that for the polymerase complex. These observations strongly suggest that the presence of EBV DNA polymerase accessory protein, BMRF1 gene product, does influence the enzymatic properties of EBV DNA polymerase catalytic subunit.
Full text
PDF
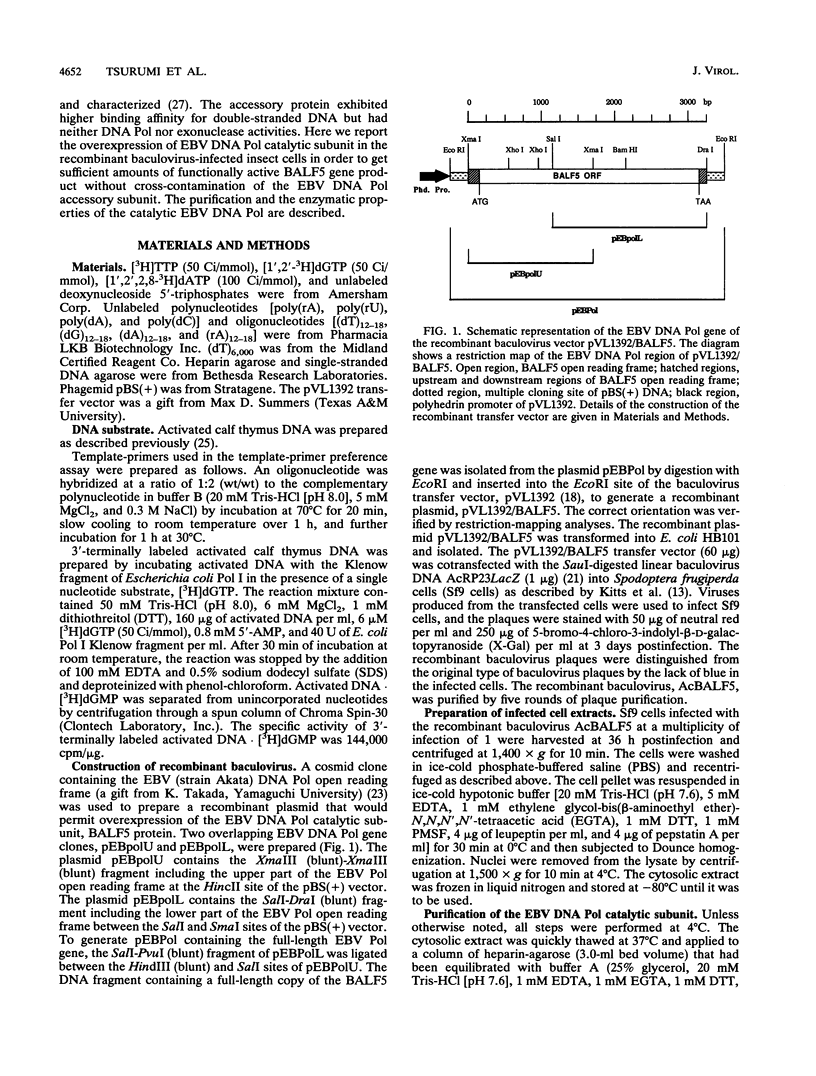

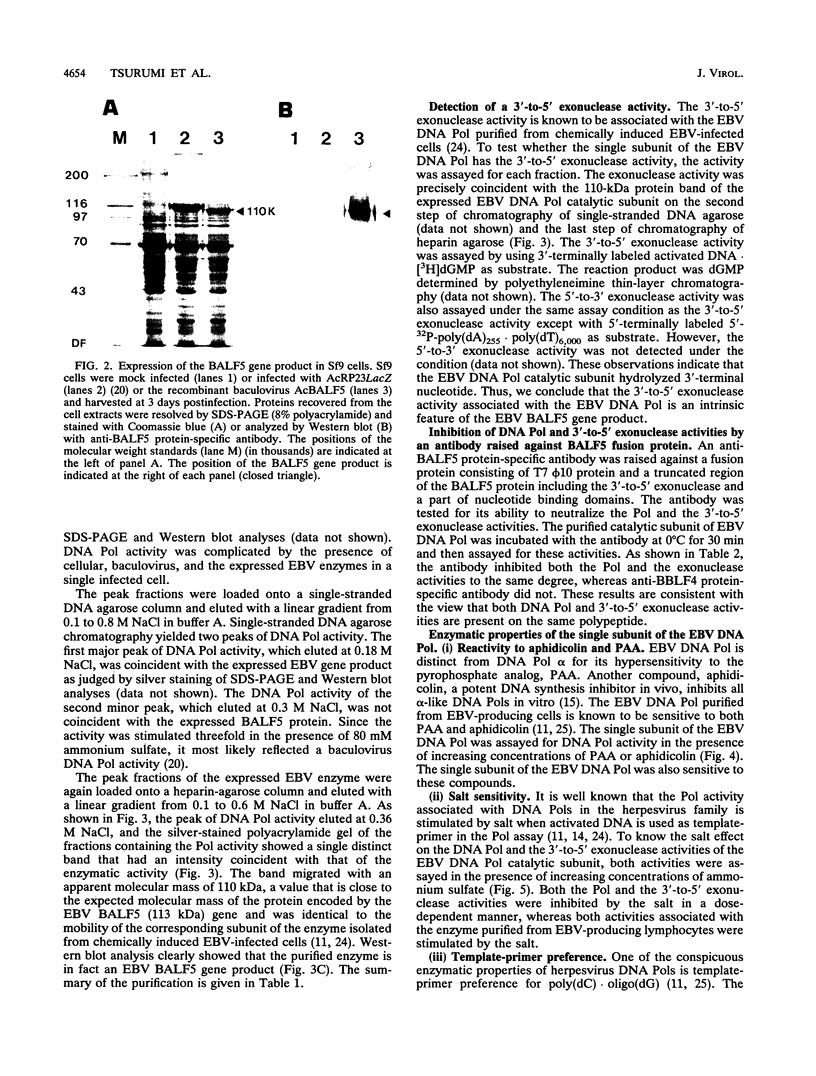

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Chiou J. F., Li J. K., Cheng Y. C. Demonstration of a stimulatory protein for virus-specified DNA polymerase in phorbol ester-treated Epstein-Barr virus-carrying cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5728–5731. doi: 10.1073/pnas.82.17.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W. C., Wang T. S. Catalytic subunit of human DNA polymerase alpha overproduced from baculovirus-infected insect cells. Structural and enzymological characterization. J Biol Chem. 1991 Nov 25;266(33):22739–22748. [PubMed] [Google Scholar]
- Crute J. J., Lehman I. R. Herpes simplex-1 DNA polymerase. Identification of an intrinsic 5'----3' exonuclease with ribonuclease H activity. J Biol Chem. 1989 Nov 15;264(32):19266–19270. [PubMed] [Google Scholar]
- Ertl P. F., Thomas M. S., Powell K. L. High level expression of DNA polymerases from herpesviruses. J Gen Virol. 1991 Jul;72(Pt 7):1729–1734. doi: 10.1099/0022-1317-72-7-1729. [DOI] [PubMed] [Google Scholar]
- Fixman E. D., Hayward G. S., Hayward S. D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992 Aug;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A. I., Coen D. M., Challberg M. D. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990 Dec;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Kallin B., Sternås L., Saemundssen A. K., Luka J., Jörnvall H., Eriksson B., Tao P. Z., Nilsson M. T., Klein G. Purification of Epstein-Barr virus DNA polymerase from P3HR-1 cells. J Virol. 1985 May;54(2):561–568. doi: 10.1128/jvi.54.2.561-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl A., Dorsky D. I. Cooperation of EBV DNA polymerase and EA-D(BMRF1) in vitro and colocalization in nuclei of infected cells. Virology. 1991 Sep;184(1):330–340. doi: 10.1016/0042-6822(91)90849-7. [DOI] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Li J. S., Zhou B. S., Dutschman G. E., Grill S. P., Tan R. S., Cheng Y. C. Association of Epstein-Barr virus early antigen diffuse component and virus-specified DNA polymerase activity. J Virol. 1987 Sep;61(9):2947–2949. doi: 10.1128/jvi.61.9.2947-2949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Sista N. D., Besençon F., Kamine J., Pagano J. S. Identification and functional characterization of Epstein-Barr virus DNA polymerase by in vitro transcription-translation of a cloned gene. J Virol. 1991 May;65(5):2728–2731. doi: 10.1128/jvi.65.5.2728-2731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Olivo P. D., Challberg M. D., Coen D. M. Enzymatic activities of overexpressed herpes simplex virus DNA polymerase purified from recombinant baculovirus-infected insect cells. Nucleic Acids Res. 1990 Mar 11;18(5):1207–1215. doi: 10.1093/nar/18.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Jewell J. E., Browne D. Baculovirus induction of a DNA polymerase. J Virol. 1981 Oct;40(1):305–308. doi: 10.1128/jvi.40.1.305-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D., Howard S. C. Analysis of the polyhedrin gene promoter of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1987 Dec 23;15(24):10233–10248. doi: 10.1093/nar/15.24.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Takada K., Horinouchi K., Ono Y., Aya T., Osato T., Takahashi M., Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991 Apr;5(2):147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- Tsurumi T. Characterization of 3'-to 5'-exonuclease activity associated with Epstein-Barr virus DNA polymerase. Virology. 1991 May;182(1):376–381. doi: 10.1016/0042-6822(91)90685-5. [DOI] [PubMed] [Google Scholar]
- Tsurumi T. Primer terminus recognition and highly processive replication by Epstein-Barr virus DNA polymerase. Biochem J. 1991 Dec 15;280(Pt 3):703–708. doi: 10.1042/bj2800703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi T. Purification and characterization of the DNA-binding activity of the Epstein-Barr virus DNA polymerase accessory protein BMRF1 gene products, as expressed in insect cells by using the baculovirus system. J Virol. 1993 Mar;67(3):1681–1687. doi: 10.1128/jvi.67.3.1681-1687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi T. Selective inhibition of the 3'-to-5' exonuclease activity associated with Epstein-Barr virus DNA polymerase by ribonucleoside 5'-monophosphates. Virology. 1992 Aug;189(2):803–807. doi: 10.1016/0042-6822(92)90611-r. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. C., Reddy M. K., von Hippel P. H. Structure and function of the bacteriophage T4 DNA polymerase holoenzyme. Biochemistry. 1992 Sep 22;31(37):8675–8690. doi: 10.1021/bi00152a001. [DOI] [PubMed] [Google Scholar]