Abstract
Mood disorders are among the most common neuropsychiatric illnesses, yet little is known about their neurobiology. Recent neuroimaging studies have found that the volume of the subgenual part of Brodmann’s area 24 (sg24) is reduced in familial forms of major depressive disorder (MDD) and bipolar disorder (BD). In this histological study, we used unbiased stereological techniques to examine the cellular composition of area sg24 in two different sets of brains. There was no change in the number or size of neurons in area sg24 in mood disorders. In contrast, the numbers of glia were reduced markedly in both MDD and BD. The reduction in glial number was most prominent in subgroups of subjects with familial MDD (24%, P = 0.01) or BD (41%, P = 0.01). The glial reduction in subjects without a clear family history was lower in magnitude and not statistically significant. Consistent with neuroimaging findings, cortical volume was reduced in area sg24 in subjects with familial mood disorders. Schizophrenic brains studied as psychiatric controls had normal neuronal and glial numbers and cortical volume. Glial and neuronal numbers also were counted in area 3b of the somatosensory cortex in the same group of brains and were normal in all psychiatric groups. Glia affect several processes, including regulation of extracellular potassium, glucose storage and metabolism, and glutamate uptake, all of which are crucial for normal neuronal activity. We thus have identified a biological marker associated with familial mood disorders that may provide important clues regarding the pathogenesis of these common psychiatric conditions.
Although the biological mechanisms that underlie mood disorders (major depressive disorder, or MDD, and bipolar disorder, or BD) are unknown, recent neuroimaging studies have revealed structural and functional changes in depressed subjects in a circuit including the prefrontal cortex (PFC), amygdala, mediodorsal thalamus, and ventromedial striatum (1). In addition to areas of increased blood flow (2), Drevets et al. (3) have found that the medial PFC ventral to the genu of the corpus callosum had abnormally reduced blood flow and metabolism in familial cases of MDD and BD (demonstrated with positron emission tomography). This was at least partly accounted for by a predominantly left-lateralized reduction in gray matter volume (demonstrated with MRI). This finding has been replicated in familial mood disordered subjects but not in subjects with nonfamilial illness (4).
The ventromedial PFC is of particular interest with respect to the pathogenesis of mood disorders because of its connections with the amygdala, hypothalamus, and midbrain periaqueductal gray, structures that have been implicated in emotional behavior and responses to stress (1, 5, 6). Lesions of the ventromedial PFC in humans lead to an inability to react viscerally to emotionally significant stimuli (7). Thus, abnormal ventromedial PFC function could underlie the alterations in emotional processing as well as the neurovegetative and endocrine changes that accompany mood disorders.
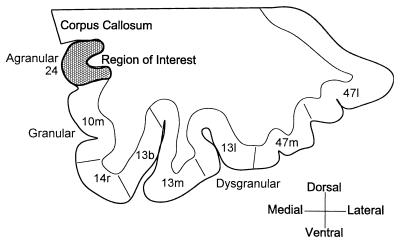
We have investigated the subgenual prefrontal cortex in a postmortem histological study by using unbiased stereological techniques. The abnormality reported by Drevets et al. (3) was localized to the anterior cingulate gyrus situated ventral to the genu of the corpus callosum. This cortex corresponds to the subgenual part of Brodmann’s area 24 (sg24) (Fig. 1). Because area sg24 is agranular, it readily is distinguished from the granular cortex ventral to it on the medial wall. Measurements on this architectonically defined area were compared with measurements on area 3b in the primary somatosensory cortex from the same brains.
Figure 1.
Coronal section through the ventral prefrontal cortex showing the cytoarchitectonic divisions on the medial wall and orbital surface. The region of interest in this study is shaded. The areal designations are derived from the description of this region in monkeys (26).
METHODS AND MATERIALS
Human brain specimens were obtained from two brain banks. Because of differences in the methods of collection and classification of tissue, in the amount of clinical documentation available, and in factors such as age, the two groups of specimens were treated as separate samples and were analyzed separately.
Tissue blocks of the ventral prefrontal cortex obtained from the Harvard Brain Tissue Resource Center (HBTRC) (Cambridge, MA) were stored in 4% paraformaldehyde and 15% glycerin (see Table 1 for demographic data). Left hemisphere blocks from one control, four BD, and four MDD patients were used. These were supplemented by left-sided blocks from four additional control cases obtained from the Washington University Department of Pathology. Psychiatric diagnoses and case records were provided by the HBTRC. Based on these records, a psychiatrist (W.C.D.) blind to the microscopic assessments selected subjects with a primary mood disorder (the onset of abnormal mood episodes antedated other psychiatric or medical disorders associated with depression, such as alcoholism or cerebrovascular disease) and with evidence that the mood disorder was familial (MDD subjects with at least one first degree relative, i.e., a parent, sibling, or offspring, with either definite or probable MDD or BD, or BD subjects with at least one first degree relative with BD) (8). These cases are referred to as familial MDD (fMDD) or familial BD (fBD). Those cases not meeting criteria for familial mood disorders were classified into MDD-other (oMDD) or BD-other (oBD) categories. Although some of these subjects also may have had familial illness, too little information was available to establish a clear diagnosis in possibly affected relatives (e.g., see legend for Fig. 2).
Table 1.
Demographic and histological data from area sg24
| Volume, mm3 | Neuronal density, cells/mm3, ×103 | Neuronal number, ×106 | Glial density, cells/mm3, ×103 | Glial number, ×106 | Age at death | Sex | PMI, hr | Time in fixative, month | |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 5) | 214.7 ± 32.8 | 25.0 ± 1.6 | 5.24 ± 0.87 | 41.7 ± 3.5 | 8.43 ± 0.97 | 72.4 ± 8.0 | 1F, 4M | 13.1 ± 3.8 | 16 ± 6 |
| MDD (n = 4) | 177.9 ± 27.5 | 34.5 ± 5.4 | 5.97 ± 1.10 | 21.5* ± 7.2 | 3.90* ± 1.45 | 59.5 ± 12.2 | 1F, 3M | 7.2 ± 4.8 | 110* ± 12 |
| BD (n = 4) | 170.9 ± 34.6 | 26.1 ± 4.0 | 4.04 ± 1.64 | 25.9* ± 8.2 | 4.83 ± 2.05 | 54.8 ± 12.6 | 2F, 2M | 18.8 ± 18.8 | 64 ± 22 |
All samples were from the left hemisphere. Brains were from HBTRC and Washington University. F, female; M, male; PMI, postmortem interval in hours; Pearson correlation coefficient between time in fixative and volume r = −0.29.
P < 0.05.
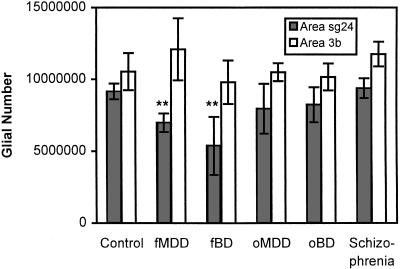
Figure 2.
Mean glial number estimates and standard errors in areas sg24 and 3b for the brains obtained from the Stanley Foundation (∗∗ indicates P = 0.01 compared with the control group). Group abbreviations fMDD and fBD indicate cases with a clear family history of the disorder while oMDD and oBD indicate cases without a clear family history. Note that the oMDD and oBD groups may include familial cases where there was not sufficient evidence to clearly establish a family history of mood disorders. For example, two of the oBD subjects had very low glial counts. Of these, one had first degree relatives with alcoholism, obsessive–compulsive disorder, and depression while the other had negative family history, but the paternal side of the family was said to be “hyperactive” and had “lots of energy.” These descriptions of the relatives are potentially compatible with a bipolar pedigree (8).
The groups were matched in age, sex, and postmortem interval, although the storage time in fixative was significantly shorter for control brains than for mood disordered groups. The blocks were frozen in a mixture of isopentane and dry ice and were cut into 20 series of 50-μm-thick sections on a sliding microtome. One series, beginning at a random position in the tissue block, was stained with the Nissl method. The area studied (area sg24) was delimited dorsally by the corpus callosum and ventrally by the transition from agranular to granular cortex. This area generally corresponded to the first full subgenual gyrus. The anterior limit for the region of interest was the first section with the corpus callosum. Eight sections were used from each brain; the posterior limit was approximately at the section where the putamen is separated from the caudate nucleus by the internal capsule. For all stereology procedures, a personal computer and microscope equipped with the Computer Assisted Stereological Toolbox-Grid system (Olympus, Albertslund, Denmark) was used. The Cavalieri principle was used to calculate the volume of the region of interest: Areas from all outlined sections were summed and then multiplied by the distance between sections (1,000 μm). Cell numbers were estimated by using the optical dissector method, which avoids double counting and makes no assumptions about cell size or shape (9). An optical dissector depth of 12 μm was used with guard volumes on top and bottom. During the uniform random sampling procedure at high magnification, an average of 10 fields was counted on each section, resulting in an average of 80 fields. Neurons and glia falling in the dissector volume were recorded separately. An average of 203 ± 8 neurons and 312 ± 15 glia were counted for each brain (mean ± standard error), well in excess of the 100–150 counts needed to achieve reliable estimates by using the optical dissector (9). Neurons were distinguished by their larger size, nonspherical shape, stained cytoplasm, and a nucleolus. Glial nuclei were small and round with no stained cytoplasm. No attempt was made to subdivide glia types based on their profiles in the Nissl stained tissue. The total number of each cell type counted was divided by the volume sampled (no. of stops × shrinkage factor × dissector depth × dissector area) to arrive at the volumetric cell density. The shrinkage factor is the thickness at which the sections were cut divided by the measured thickness of the sections on the slides. The cell density then was multiplied by the calculated volume of the region of interest to give the total number of cells in that region. Intergroup differences in volume were assessed by 1-tailed t tests whereas glial numbers were compared by using 2-tailed t tests. This preliminary study was described partially (without the analysis of family history) (10).
In a separate study, 59 brains (15 controls, 15 schizophrenics, 15 BD cases, and 14 MDD cases) were obtained from the Stanley Foundation (Bethesda, MD). Case summaries were provided with information about illness course, medication history, substance abuse, and family history (Table 2). Examinations of the brains by two different neuropathologists revealed no signs of Alzheimer’s disease or other pathological conditions. There were no significant differences between the groups in age, sex, and education level. All subjects included in the analysis were white except for one black subject in the oBD group. The suddenness of death, postmortem interval, and time in fixative were slightly greater in the psychiatric cases than in controls, but the difference was significant only for the fBD group for suddenness of death and for the BD, oBD, and schizophrenia groups for time in fixative. There were no significant differences between the mood disordered and schizophrenic groups in these measures. Brain pH, an indicator of tissue preservation, was similar across groups (mean measures: 6.1 for controls, 6.3 for schizophrenics, 6.2 for BD, and 6.2 for MDD cases), and mRNA yield was excellent or good in almost all cases. Using the family history information based on reports by family members, a psychiatrist (W.C.D.) blind to the histopathological measurements subclassified subjects into those who had a probable family history of MDD or BD and those whose family history was negative or indeterminate.
Table 2.
Demographic data on the brains from the Stanley Foundation
| Age at death | Sex | Education, years | Age of onset | No. of suicides | Suddenness of death | PMI, hr | Time in fixative, mo | Hemisphere | |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 11) | 49.1 ± 3.4 | 4F, 7M | 15.0 ± 0.5 | — | 0 | 1.1 ± 0.1 | 23.1 ± 2.9 | 16.7 ± 2.4 | 5 R, 6 L |
| MDD (n = 9) | 47.3 ± 3.0 | 4F, 5M | 14.4 ± 0.5 | 30.2 ± 4.7 | 4 | 1.4 ± 0.2 | 24.7 ± 3.0 | 21.4 ± 3.1 | 4 R, 5 L |
| fMDD (n = 6) | 42.8 ± 3.7 | 3F, 3M | 14.2 ± 0.7 | 27.5 ± 4.3 | 3 | 1.4 ± 0.2 | 25.0 ± 5.3 | 19.0 ± 4.2 | 4 R, 2 L |
| oMDD (n = 3) | 56.3 ± 3.8 | 1F, 2M | 15.0 ± 0.6 | 35.7 ± 12.4 | 1 | 1.3 ± 0.3 | 33.0 ± 4.9 | 26.3 ± 3.4 | 3 L |
| BD (n = 14) | 44.9 ± 3.2 | 5F, 9M | 13.3 ± 0.7 | 22.9 ± 2.6 | 6 | 1.8 ± 0.2 | 30.6 ± 4.0 | 26.6 ± 1.2* | 7 R, 7 L |
| fBD (n = 4) | 49.0 ± 7.0 | 3F, 1M | 12.3 ± 1.7 | 19 ± 3.8 | 3 | 2.0 ± 0.4* | 40.0 ± 7.9 | 26.0 ± 2.2 | 2 R, 2 L |
| oBD (n = 10) | 43.2 ± 3.4 | 2F, 8M | 13.8 ± 0.6 | 24.9 ± 3.3 | 3 | 1.8 ± 0.3 | 26.8 ± 4.0 | 26.9 ± 1.5* | 5 R, 5 L |
| Schizophrenia (n = 11) | 39.7 ± 3.9 | 3F, 7M | 12.8 ± 0.7 | 21.8 ± 2.2 | 4 | 1.8 ± 0.2 | 34.1 ± 5.4 | 27.6 ± 2.0* | 5 R, 6 L |
Abbreviations as in Table 1; R, right; L, left. Suddenness of death was recorded on a scale of 1 to 5 where 1 was instantaneous and 5 was very slow with possible hypoxia.
P < 0.05.
The primary diagnostic classifications assigned by the Stanley Foundation review committee were accepted in all cases except one. This subject’s case summary documented the clinical features required to meet DSM-IV criteria for schizoaffective disorder and reported a family history remarkable for one sibling and one offspring diagnosed with BD, one offspring who suffered from depression, and one sibling diagnosed as having schizophrenia. The case summary listed the differential diagnosis as including schizoaffective disorder and schizophrenia, reflecting the limitations of psychiatric diagnostic procedures in the absence of objective biological criteria, although the Stanley Foundation review committee favored the diagnosis of schizophrenia. This individual was classified by W.C.D. into the fBD group for the purposes of the current study, in keeping with the evidence that the putative bipolar genotype may be manifested as the phenotypes of schizoaffective disorder, BD, or MDD (8).
Among the brains that were included in the analysis (see below for exclusion criteria), no MDD subject had ever been psychotic. Three of four fBD and 7 of 10 oBD subjects had a history of developing psychotic features at some point during their affective illness. Three controls, three MDD subjects, 10 BD subjects, and four schizophrenics had been substance abusers either at time of death or at an earlier time. The only subject to receive electroconvulsive therapy was in the schizophrenic category. At the time of death, MDD subjects were receiving tricyclic (n = 4) or selective serotonin reuptake inhibitor (n = 6) antidepressants, buproprion (n = 1), nefazodone (n = 2), trazodone (n = 2), lithium (n = 2), or dilantin (n = 1). BD subjects were receiving lithium (n = 3), anticonvulsants (n = 7), bupropion (n = 2), tricyclic antidepressants (n = 1), antipsychotic agents (n = 6), or anxiolytics (n = 2). Three oBD subjects and one oMDD subject were medication free at the time of death. There was no apparent overall difference in medication history between subjects in the familial and other groups.
Tissue from only one hemisphere was available, and the side provided was random for each brain, with about equal numbers of left and right sides in each diagnostic category. Blocks from the ventral PFC were processed as above, except that 16 series of 50-μm sections were cut to increase the number of sections falling in the region of interest to 10 (resulting intersection distance was 800 μm). Because damage to superficial layers of cortex by knife-cuts and tears can lead to erroneous volume and hence cell number measurements, brains with substantial damage in area sg24 (four control, four schizophrenic, two BD, and five MDD brains) were excluded from the analysis. The anterior and posterior limits used were the same as above, but a few samples did not include tissue that spanned the full extent of these limits. In these cases, <10 sections were measured, and the resultant volume and cell numbers were scaled up to the equivalent values for 10 sections. Volume and cell number measurements were carried out blinded and in random order as above, with an optical dissector depth of 10 μm. Intergroup differences in volume, glial number, and glial density were assessed by 1-tailed t tests.
Estimations of neuronal and glial volume were made for counted cells by using the stereological rotator method (11). Because glial cytoplasm is not apparent in Nissl stained sections, only nuclear volume could be measured for glia. The rotator method assumes random sectioning axes for the cells being measured. In this case, it was found that the curvature of the cortex around the subgenual gyrus ensured that the anisotropic axes of cortical neurons were distributed randomly. Glial nuclei in the cortex are not aligned along any axis. Cell sizes were averaged for individual brains to arrive at a mean glial nuclear or neuronal volume for that subject. This number served as an indicator of overall changes in cell size across groups. In addition, cells were binned into volume ranges such as 0–500 μm3, 500–1,000 μm3, etc., and cell numbers in each range were normalized: that is, they were expressed as percentages of the range with the maximum number of cells.
For statistical analysis, the results from the first group of specimens (from the HBTRC) formed a preliminary hypothesis generating set whereas the results from the second set (from the Stanley Foundation) were considered as a hypothesis testing set. Two-tailed t tests therefore were used to test for differences in glial number with the first group whereas 1-tailed t tests were used with the second group.
The Stanley Foundation also provided tissue samples from the region of the central sulcus in the same brains, and these were processed in the same way as those from the prefrontal cortex. Area 3b, in the posterior bank of the sulcus, was distinguished by architectonic criteria, and the number and volumetric density of neurons and glia were measured in 10 sections containing this area taken from a 1-in-12 series. Because fewer brains were excluded from the analysis due to tears or cuts in the tissue, the number of subjects was larger in all groups in this portion of the study.
RESULTS
A preliminary study was conducted using brain tissue obtained from the HBTRC. Using unbiased stereologic sampling methods on these brains (five controls, four MDD, four BD), we found decreased volume in area sg24 in both MDD and BD, compared with controls. The reductions in volume (17.1% in MDD and 20.4% in BD) were not significant in either group because of the small number of brains and the variability in each group. Although the MDD and BD brains had spent a significantly longer time in fixative, this time correlated only weakly with the volume measures (Table 1).
In three BD cases with a family history of BD, the mean volume of area sg24 was 35.7% smaller than in controls (1-tailed t test gave t = 2.15, P = 0.068). This is comparable to the 38% reduction in the MRI-based volume measure of this region reported by Drevets et al. in familial BD (3). Case records were missing in two MDD cases. The remaining one BD and two MDD brains had no clear family history, and their mean area sg24 volumes were similar to those of controls.
The density and numbers of neurons and glia also were measured in area sg24 (Table 1). Mean neuronal numbers for both the MDD and BD groups were similar to those of controls. The density of neurons in the MDD and BD groups was slightly higher than control, probably because the volume of area sg24 was smaller in those groups. However, the numbers and density of glia were reduced markedly in both mood-disordered groups. Because of the large variance in glial counts, the difference in glial numbers achieved statistical significance only in the MDD group (Table 1; 2-tailed t = 2.61, P = 0.031 for MDD vs. control and t = 1.68, P = 0.131 for BD vs. control). When only the fBD cases were considered, the glial reduction was significant (2-tailed t = 2.89, P = 0.023). Preliminary observations also indicated that the glial reduction also was found in the posteromedial orbital cortex in both MDD and BD cases. The small number of subjects in this preliminary study necessitated that these observations be confirmed in a larger subject group.
In the second tissue set, provided by the Stanley Foundation, the groups with familial mood disorders had greater degrees of reduced volume in area sg24 than the oMDD and oBD groups, although the differences with respect to controls were not statistically significant (12.4% volume loss in fMDD, P = 0.164 vs. 7.4% increase in volume in oMDD; 23.5% loss in fBD, P = 0.092 vs. 1.3% loss in oBD; 1-tailed t tests) (Table 3). Because this study included brains from both the left and the right hemispheres, the volumetric measures are not directly comparable to those from the imaging study of Drevets et al. (3), in which the volumetric reductions were left lateralized. To account for differences in whole brain size, the volume measurements on area sg24 were divided by whole wet brain weight measures taken at autopsy. This correction was similar across groups, indicating that volumetric changes are not caused by global brain atrophy in subjects with affective disorder (Table 3).
Table 3.
Weight and histological data from area sg24
| Whole brain weight, g | Volume of area sg24, mm3 | Volume/ whole brain weight, mm3/g | Neuronal density, cells/mm3, ×103 | Neuronal number, ×106 | Glial density, cells/mm3, ×103 | Glial number, ×106 | |
|---|---|---|---|---|---|---|---|
| Control (n = 11) | 1,470.5 ± 50.9 | 203.9 ± 15.0 | 0.140 ± 0.012 | 25.3 ± 1.4 | 5.22 ± 0.57 | 45.7 ± 2.3 | 9.15 ± 0.55 |
| MDD (n = 9) | 1,443.3 ± 45.6 | 192.0 ± 17.4 | 0.135 ± 0.013 | 26.8 ± 1.8 | 5.17 ± 0.53 | 38.8* ± 3.3 | 7.3* ± 0.89 |
| fMDD (n = 6) | 1,433.3 ± 62.4 | 178.6 ± 24.2 | 0.127 ± 0.017 | 25.3 ± 3.5 | 4.44 ± 0.54 | 39.7* ± 2.2 | 6.97† ± 0.80 |
| oMDD (n = 3) | 1,463.3 ± 72.2 | 218.9 ± 24.3 | 0.151 ± 0.022 | 29.7 ± 1.8 | 6.62 ± 1.07 | 37.1 ± 6.5 | 7.95 ± 1.51 |
| BD (n = 14) | 1,431.2 ± 46.7 | 188.3 ± 18.3 | 0.133 ± 0.012 | 27.9 ± 1.7 | 5.05 ± 0.43 | 37.7* ± 3.1 | 7.42 ± 1.10 |
| fBD (n = 4) | 1,406.3 ± 99.2 | 155.9 ± 40.9 | 0.108 ± 0.025 | 29.3 ± 1.8 | 4.45 ± 1.10 | 33.9* ± 7.2 | 5.38† ± 2.00 |
| oBD (n = 10) | 1,442.2 ± 55.0 | 201.2 ± 18.4 | 0.144 ± 0.014 | 27.4 ± 2.2 | 5.29 ± 0.41 | 39.2 ± 3.3 | 8.23 ± 1.22 |
| Schizophrenia (n = 11) | 1,499.0 ± 37.7 | 224.8 ± 16.9 | 0.149 ± 0.010 | 25.2 ± 1.4 | 5.59 ± 0.47 | 42.1 ± 1.5 | 9.38 ± 0.66 |
Brains were from the Stanley Foundation.
P ≤ 0.05.
P ≤ 0.01.
The pattern of changes in glial cells was similar to that seen in the preliminary study. Although glial counts showed substantial variability within each group, the mean glial number in the MDD group as a whole was significantly reduced in area sg24 compared with the control group (20.2% reduction, t = 2.47, P = 0.023; 1-tailed t test) (Table 3). This effect was caused mainly by changes in MDD subjects with a family history of depression (23.8% reduction, t = 2.76, P = 0.014 in fMDD vs. 13.1% reduction; t = 1.36, P = 0.197 in oMDD; 1-tailed t tests). The glial numbers for BD subjects were significantly reduced in fBD but not in oBD with respect to controls (41.2% reduction, t = 2.91, P = 0.011 in fBD vs. 10.0% reduction; t = 1.20, P = 0.244 in oBD; 1-tailed t tests) (Table 3 and Fig. 2). A similar pattern of changes was detected in glial density measures. Preliminary separate measurements on the superficial and deep layers of cortex in area sg24 suggest that the glial reductions in fMDD and fBD are not restricted to specific cortical layers.
Glial nuclear size measurements did not show any mean differences across groups in area sg24 (data not shown). An analysis of the distribution of glia across size bins indicated that, in the fBD cases, there was a relative decrease in glial cells with nuclear sizes between 75 and 150 μm3 (30.7% fewer glia; 2-tailed t = 3.01, P = 0.009). However, the distribution of glia nuclear sizes in the fMDD group was very similar to that in the control group.
In contrast to glial number and density, measurements of neuronal density, number, and size in the subgenual prefrontal cortex revealed no abnormalities in mood disordered brains. Schizophrenics were not different from controls in either volume or in glial number in area sg24, but they did show a subtle abnormality in neuronal size distribution. There were significantly fewer cells in the volume ranges of 2,500–3,000 μm3 and 3,000–3,500 μm3 compared with control (P = 0.030 and P = 0.025, respectively) and more cells with volumes between 1,000 and 1,500 μm3 (P = 0.054). Coupled with normal neuronal numbers in area sg24, this suggests a shrinkage of large neurons in schizophrenia and is qualitatively similar to the effect observed by Rajkowska et al. in the dorsolateral prefrontal cortex (12).
Measurements of neuronal and glial number also were carried out in an unrelated area of cortex, somatosensory-related area 3b on the posterior bank of the central sulcus. In contrast to the pattern of findings in the prefrontal cortex, there were no significant differences in area 3b between the groups on any measure, including glial density and number in this area (Table 4; Fig. 2).
Table 4.
Histological data from somatosensory area 3b
| Volume, mm3 | Neuronal density, cells/mm3, ×103 | Neuronal number, ×106 | Glial density, cells/mm3, ×103 | Glial number, ×106 | |
|---|---|---|---|---|---|
| Control (n = 13) | 186.9 ± 19.0 | 34.4 ± 1.2 | 6.38 ± 0.60 | 55.9 ± 2.4 | 10.54 ± 1.28 |
| MDD (n = 11) | 186.4 ± 10.7 | 38.7 ± 1.2 | 7.24 ± 0.46 | 59.5 ± 2.9 | 11.22 ± 1.03 |
| fMDD (n = 5) | 192.5 ± 21.0 | 36.5 ± 1.8 | 7.02 ± 0.77 | 67.0 ± 5.0 | 12.09 ± 2.16 |
| oMDD (n = 6) | 181.4 ± 10.7 | 40.6 ± 1.6 | 7.41 ± 0.66 | 58.5 ± 4.1 | 10.50 ± 0.62 |
| BD (n = 15) | 168.7 ± 9.3 | 37.9 ± 1.2 | 6.37 ± 0.40 | 60.1 ± 3.1 | 10.04 ± 0.74 |
| fBD (n = 5) | 172.6 ± 20.0 | 35.5 ± 3.0 | 5.98 ± 0.38 | 59.0 ± 9.2 | 9.80 ± 1.52 |
| oBD (n = 10) | 166.8 ± 11.4 | 39.1 ± 1.2 | 6.57 ± 0.57 | 60.6 ± 2.6 | 10.17 ± 0.93 |
| Schizophrenia (n = 12) | 186.7 ± 12.0 | 36.9 ± 1.2 | 6.88 ± 0.48 | 62.6 ± 1.6 | 11.76 ± 0.86 |
Brains were from the Stanley Foundation.
DISCUSSION
Using brain tissue from two independent sources, we found a selective and significant reduction of glial cell number and density in area sg24 in subjects with familial mood disorders. This reduction was associated with a loss of volume in area sg24 similar to that found by using neuroimaging measures by Drevets et al. (3). Although it remains unclear how much of the volumetric abnormality can be accounted for by the observed glial reduction, the volume reduction in area sg24 clearly was not associated with changes in neuronal somatic size or number (dendritic and axonal changes were not assessed).
The subdivision of mood disordered groups into familial and other categories was based on reports by family members. This family history method is subject to the limitations that the family members providing the information may have been misinformed by their affected relatives or may have obtained erroneous or outdated diagnoses from their relatives’ physicians. These limitations are expected to increase the variability of the histopathological results for the familial BD and MDD groups but would not be expected to increase the risk for Type I error. Inaccuracies in family history would in fact reduce the magnitude of measured differences between the “familial” and “other” mood disordered groups because some subjects reported to have either negative or ambiguous family histories (included together in the other groups) may have had affected relatives.
The finding of glial reduction in familial mood disorders does not appear to be related to gender or age effects or to reflect global atrophy. Effects of psychotropic medications or substance abuse cannot be ruled out because our sample did not include sufficient numbers of medication free subjects with no history of substance abuse. However, several lines of evidence indicate that these factors cannot account for the finding of reductions in glial number in familial mood disorders. First, the majority of subjects in the fMDD group typically were receiving fluoxetine and/or tricyclic antidepressants whereas fBD subjects typically were receiving lithium and/or anticonvulsants, but both groups showed reductions in glial number. Second, there was no obvious difference in the classes or number of drugs taken by subjects in the fMDD vs. oMDD and the fBD vs. oBD groups. Third, two of three MDD and 7 of 10 BD subjects with a history of substance abuse were in the nonfamilial categories, making it unlikely that substance abuse is a causal factor in glial number reduction. Finally, the fact that the glial reduction is not seen in an unrelated cortical area in the somatosensory cortex indicates that the changes are not global. It is possible but unlikely that nonspecific factors would lead to localized changes in the brain. Because no reduction in glia was evident in the schizophrenic group, this finding also cannot be a nonspecific effect related to psychiatric illness, psychosis, or antipsychotic agents.
Similar observations of glial reduction in brains from depressive subjects have been made by Rajkowska et al. (ref. 13; G. Rajkowska, J. J. Miguel-Hidalgo, J. Wei, S. D. Pittman, G. Dilley, J. Overholser, H. Meltzer, and C. Stockmeier, personal communication) in a simultaneous but independent study of the PFC. The results of this study and preliminary observations in the present study indicate that the glial reduction is found in other parts of the PFC outside area sg24. However, we have found that this reduction does not generalize to the somatosensory cortex. More work is needed to define the full extent of cortical and subcortical areas in which glial reductions are seen in mood disorders. The location of the glial reduction in areas of the PFC in which specific functional and anatomical changes in depression have been identified with neuroimaging methods (2, 13), the lack of glial changes in area sg24 in patients with nonfamilial mood disorders and with schizophrenia, and the fact that the glial changes do not appear to be caused by gender, age, medication, or substance abuse effects all suggest an intriguing relationship between glial reduction and familial mood disorders. However, it is not clear if the biological change we have identified is causally related to the psychopathology.
Glia constitute the majority of cells in the central nervous system, and the three major subtypes of glia are implicated in different functions. It is unclear at this stage whether a specific neuroglial subtype is involved in the changes we observed in depression. However, a selective change in the percentage of glial cell nuclei sized 75–150 μm3 in the fBD group compared with controls indicates that a specific class of glial cells may be altered in that condition. Detailed analyses of the distribution and function of oligodendrocytes and microglia, as well as astrocytes, are needed to understand the biological relationship between glial changes and mood disorders. For example, astrocytes are essential for maintaining potassium homeostasis in the extracellular fluid, for coupling of neuronal activity and energy metabolism, and for the uptake of synaptically released glutamate (15). Lowered capacity to buffer the excess extracellular potassium released during neuronal activity would be expected to increase neuronal depolarization, potentially resulting in increased neuronal activity and functional changes. Glia also express receptors for a number of neurotransmitters, including the serotonin 1A receptor, and may be involved in neurotransmission mechanisms within the cortex (16). Finally, glial cells play a crucial role in neuronal migration during the development of the cerebral cortex (17).
Because glial cells are able to reverse their mature phenotype and proliferate under a variety of circumstances in vivo, the numbers of glia in the central nervous system may fluctuate based on developmental conditions and throughout life. A number of factors have been reported to impact on glial proliferation, including functional activation of the cerebral cortex during development, neural cell adhesion molecules, leukemia inhibiting factor receptor, and glutamate (18, 19, 20, 21, 22). Alterations in these or other factors may underlie the phenotype we have observed.
Subjects with primary mood disorders who also have a mood-disordered first degree relative may constitute a homogeneous group with clear biological traits (23). For example, a recent MRI study that confirmed the volume loss in the subgenual PFC reported by Drevets et al. (3) in familial MDD and BD also reported that this change was not seen in subjects with nonfamilial illness (4). The apparent restriction of glial reduction in our study to familial cases of mood disorder supports this conclusion. Indeed, this specificity indicates that the glial reduction may have a heritable basis. Glial reduction in the medial prefrontal cortex constitutes a trait that ultimately could be related to specific genetic linkages. On the other hand, nonfamilial MDD or BD can develop in the presence of normal glial numbers. This observation underscores the need to use factors such as family history to identify clinical subtypes of mood disorders that can be studied and treated separately.
The cellular changes we report extend the observations of Drevets et al. that area sg24 has decreased metabolism and blood flow and has decreased volume in mood disorders (3). However, it would be premature to postulate a single mechanism leading from glial reduction to psychopathology. As noted above, similar glial changes have been observed in the orbital prefrontal cortex, a region in which metabolism and blood flow increases have been reported in MDD (24). Thus, the alterations in glia in mood disorders are not limited to regions with decreased activity. A recent study found that treatment-responsive MDD subjects were distinguished from treatment-nonresponsive MDD subjects by having higher- vs. lower-than-normal levels of glucose metabolism, respectively, in parts of the anterior cingulate cortex adjacent to area sg24 (25). Whatever role glial reduction in the prefrontal cortex has in the pathogenesis of depression, it presumably interacts with other factors, including afferent neural activity or neuromodulators such as serotonin, noradrenaline, and dopamine to produce specific disturbances in brain activity, mood and behavior.
Acknowledgments
The authors thank Jagruti Parghi and Mike Bowley for excellent technical assistance and Karan Randhava for assistance with editing. Postmortem brains were donated by the Harvard Brain Tissue Resource Center supported in part by U.S. Public Health Service Grant MH/NS 31862 and by the Stanley Foundation Brain Consortium courtesy of Drs. Llewellyn B. Bigelow, Juraj Cervenak, Mary M. Herman, Thomas M. Hyde, Joel E. Kleinman, José D. Paltan, Robert M. Post, E. Fuller Torrey, Maree J. Webster, and Robert H. Yolken. This work was supported by National Institutes of Health Grants DC00093, MH51137, and MH00928 and the Charles A. Dana Foundation.
ABBREVIATIONS
- MDD
major depressive disorder
- BD
bipolar disorder
- PFC
prefrontal cortex
- sg24
subgenual part of Brodmann’s area 24
- fMDD
familial MDD
- fBD
familial BD
- oMDD
MDD-other
- oBD
BD-other
- HBTRC
Harvard Brain Tissue Resource Center
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Price J L, Carmichael S T, Drevets W C. In: Progress in Brain Research: The Emotional Motor System. Holstege G, Bandler R, Saper C B, editors. Vol. 107. Amsterdam: Elsevier Science; 1996. pp. 523–536. [DOI] [PubMed] [Google Scholar]
- 2.Drevets W C, Videen T O, Price J L, Preskorn S H, Carmichael S T, Raichle M E. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drevets W C, Price J L, Simpson J R, Todd R D, Vannier M, Raichle M E. Nature (London) 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 4.Hirayasu, Y., Shenton, M. E., Salisbury, D. F., Wible, C. G., Fischer, I. A., Kisler, T., Kwon, J. S., Dickey, C. C., Yurgelun-Todd, D. A., Tohen, M., et al. (1998) Biol. Psychiatry 43, Suppl. 97S. [DOI] [PubMed]
- 5.An X, Bandler R, Öngür D, Price J L. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 6.Öngür D, An X, Price J L. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 7.Bechara A, Damasio H, Tranel D, Damasio A R. Science. 1997;275:1269–1272. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 8.Drevets W C, Todd R D. In: Adult Psychiatry. Guze S B, editor. St. Louis: Mosby; 1997. pp. 99–141. [Google Scholar]
- 9.Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard I R, Pakkenburg B, Sørensen F B, Vesterby A, et al. Acta Pathol Microbiol Immunol Scand. 1987;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 10.Drevets W C, Öngür D, Price J L. Mol Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 11.Tandrup T, Gundersen H J, Jensen E B. J Microsc (Oxford) 1997;186:108–120. doi: 10.1046/j.1365-2818.1997.2070765.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajkowska G, Selemon L S, Goldman-Rakic P S. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 13.Rajkowska G, Selemon L D, Goldman-Rakic P S. Schizophr Res. 1997;24:41. (abstr.). [Google Scholar]
- 14.Bench C J, Friston K J, Brown R G, Frackowiak R S, Dolan R J. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 15.Tsacopoulos M, Magistretti P J. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henn F. Prog Brain Res. 1982;55:241–252. doi: 10.1016/S0079-6123(08)64201-6. [DOI] [PubMed] [Google Scholar]
- 17.Rakic P. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 18.Diamond M C, Law F, Rhodes H, Lindner B, Rosenzweig M R, Krech D, Bennett E L. J Comp Neurol. 1967;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- 19.Szeligo F, Leblond C P. J Comp Neurol. 1977;172:247–264. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- 20.Krushel L A, Tai M-H, Cunningham B A, Edelman G A, Crossin K L. Proc Natl Acad Sci USA. 1998;95:2592–2596. doi: 10.1073/pnas.95.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koblar S A, Turnley A M, Classon B J, Reid K L, Ware C B, Cheema S S, Murphy M, Bartlett P F. Proc Natl Acad Sci USA. 1998;95:3178–3181. doi: 10.1073/pnas.95.6.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti F, Magri G, Ingrao F, Bruno V, Catania M V, Dell’Albani P, Condorelli D F, Avola R. J Neurochem. 1990;54:771–777. doi: 10.1111/j.1471-4159.1990.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 23.Drevets W C, Botteron K. In: Adult Psychiatry. Guze S B, editor. St. Louis: Mosby; 1997. pp. 53–82. [Google Scholar]
- 24.Drevets W C. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 25.Mayberg H S, Brannan S K, Mahurin R K, Jerabek P A, Brickman J S, Tekell J L, Silva J A, McGinnis S, Glass T G, Martin C C, et al. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 26.Carmichael S T, Price J L. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]