Abstract
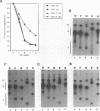
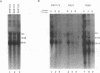
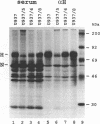
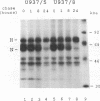
The alpha/beta (type I) interferon-inducible human MxA protein confers resistance to vesicular stomatitis virus (VSV) and influenza A virus in MxA-transfected mouse 3T3 cells (3T3/MxA). We investigated the inhibitory effects of the MxA protein on measles virus (MV) and VSV in the human monocytic cell line U937. In transfected U937 clones which constitutively express MxA (U937/MxA), the release of infectious MV and VSV was reduced approximately 100-fold in comparison with control titers. Transcription of VSV was inhibited similar to that observed for 3T3/MxA cells, whereas no difference was detected for MV in the rates of transcription or the levels of MV-specific mRNAs. In contrast, analysis of MV protein expression by immunofluorescence and immunoprecipitation revealed a significant reduction in the synthesis of MV glycoproteins F and H in U937/MxA cells. These data demonstrate a virus-specific effect of MxA which may, in the case of MV, contribute to the establishment of a persistent infection in human monocytic cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Fäh J., Hurt N., Samuel C. E., Thomis D., Bazzigher L., Pavlovic J., Haller O., Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989 Nov;9(11):5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Andjaparidze O. G., Chaplygina N. M., Bogomolova N. N., Koptyaeva I. B., Nevryaeva E. G., Filimonova R. G., Tareeva I. E. Detection of measles virus genome in blood leucocytes of patients with certain autoimmune diseases. Arch Virol. 1989;105(3-4):287–291. doi: 10.1007/BF01311364. [DOI] [PubMed] [Google Scholar]
- Baczko K., Carter M. J., Billeter M., ter Meulen V. Measles virus gene expression in subacute sclerosing panencephalitis. Virus Res. 1984 Oct;1(7):585–595. doi: 10.1016/0168-1702(84)90015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D. W., Sullivan J. L., Lucas S. J., Dunlap R. C., Albrecht P. Acute and chronic infection of human lymphoblastoid cell lines with measles virus. J Immunol. 1976 Jan;116(1):89–98. [PubMed] [Google Scholar]
- Celma M. L., Fernandez-Muñoz R. Measles virus gene expression in lytic and persistent infections of a human lymphoblastoid cell line. J Gen Virol. 1992 Sep;73(Pt 9):2203–2209. doi: 10.1099/0022-1317-73-9-2203. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fournier J. G., Tardieu M., Lebon P., Robain O., Ponsot G., Rozenblatt S., Bouteille M. Detection of measles virus RNA in lymphocytes from peripheral-blood and brain perivascular infiltrates of patients with subacute sclerosing panencephalitis. N Engl J Med. 1985 Oct 10;313(15):910–915. doi: 10.1056/NEJM198510103131502. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. Interferon-induced human protein MxA is a GTPase which binds transiently to cellular proteins. J Virol. 1992 Aug;66(8):4705–4709. doi: 10.1128/jvi.66.8.4705-4709.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Pavlovic J., Staeheli P., Krystal M. Overexpression of the influenza virus polymerase can titrate out inhibition by the murine Mx1 protein. J Virol. 1992 Jul;66(7):4154–4160. doi: 10.1128/jvi.66.7.4154-4160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyypiä T., Korkiamäki P., Vainionpä R. Replication of measles virus in human lymphocytes. J Exp Med. 1985 Jun 1;161(6):1261–1271. doi: 10.1084/jem.161.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., McFarland H. F. Measles virus persistence in human lymphocytes: a role for virus-induced interferon. J Gen Virol. 1982 Dec;63(2):351–357. doi: 10.1099/0022-1317-63-2-351. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune F., Sakata H., Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990 Feb;64(2):700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi R., Hyypiä T., Vainionpä R. Effect of interferon-alpha on measles virus replication in human peripheral blood mononuclear cells. APMIS. 1992 Feb;100(2):125–131. doi: 10.1111/j.1699-0463.1992.tb00850.x. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- Meyer T., Horisberger M. A. Combined action of mouse alpha and beta interferons in influenza virus-infected macrophages carrying the resistance gene Mx. J Virol. 1984 Mar;49(3):709–716. doi: 10.1128/jvi.49.3.709-716.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench T. R., Griffin D. E., Obriecht C. R., Vaisberg A. J., Johnson R. T. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J Infect Dis. 1988 Aug;158(2):433–442. doi: 10.1093/infdis/158.2.433. [DOI] [PubMed] [Google Scholar]
- Ogura H., Baczko K., Rima B. K., ter Meulen V. Selective inhibition of translation of the mRNA coding for measles virus membrane protein at elevated temperatures. J Virol. 1987 Feb;61(2):472–479. doi: 10.1128/jvi.61.2.472-479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H., Rima B. K., Tas P., Baczko K., ter Meulen V. Restricted synthesis of the fusion protein of measles virus at elevated temperatures. J Gen Virol. 1988 Apr;69(Pt 4):925–929. doi: 10.1099/0022-1317-69-4-925. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- PETRALLI J. K., MERIGAN T. C., WILBUR J. R. CIRCULATING INTERFERON AFTER MEASLES VACCINATION. N Engl J Med. 1965 Jul 22;273:198–201. doi: 10.1056/NEJM196507222730405. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Haller O., Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992 Apr;66(4):2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Zürcher T., Haller O., Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990 Jul;64(7):3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Hull D., Greer P., Hasel K., Berkovich A., Englund G., Bellini W., Rima B., Lazzarini R. The nucleotide sequence of the mRNA encoding the fusion protein of measles virus (Edmonston strain): a comparison of fusion proteins from several different paramyxoviruses. Virology. 1986 Dec;155(2):508–523. doi: 10.1016/0042-6822(86)90212-6. [DOI] [PubMed] [Google Scholar]
- Robertson D. A., Zhang S. L., Guy E. C., Wright R. Persistent measles virus genome in autoimmune chronic active hepatitis. Lancet. 1987 Jul 4;2(8549):9–11. doi: 10.1016/s0140-6736(87)93051-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Yoshikawa Y., Yamanouchi K., Takeda K., Sato T. Characteristics of fresh isolates of wild measles virus. Jpn J Exp Med. 1986 Apr;56(2):61–67. [PubMed] [Google Scholar]
- Schneider-Schaulies S., Kreth H. W., Hofmann G., Billeter M., Ter Meulen V. Expression of measles virus RNA in peripheral blood mononuclear cells of patients with measles, SSPE, and autoimmune diseases. Virology. 1991 Jun;182(2):703–711. doi: 10.1016/0042-6822(91)90611-e. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., Cattaneo R., Billeter M., ter Meulen V. Restriction of measles virus gene expression in acute and subacute encephalitis of Lewis rats. Virology. 1989 Aug;171(2):525–534. doi: 10.1016/0042-6822(89)90622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., ter Meulen V. Restricted expression of measles virus in primary rat astroglial cells. Virology. 1990 Aug;177(2):802–806. doi: 10.1016/0042-6822(90)90553-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991 Aug;65(8):4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Schmitz A., Jakschies D., Von Wussow P., Horisberger M. A. A whole blood immunoassay for the interferon-inducible human Mx protein. J Interferon Res. 1992 Apr;12(2):67–74. doi: 10.1089/jir.1992.12.67. [DOI] [PubMed] [Google Scholar]
- Wrzos H., Kulczycki J., Laskowski Z., Matacz D., Brzosko W. J. Detection of measles virus antigen(s) in peripheral lymphocytes from patients with subacute sclerosing panencephalitis. Arch Virol. 1979;60(3-4):291–297. doi: 10.1007/BF01317500. [DOI] [PubMed] [Google Scholar]