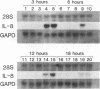
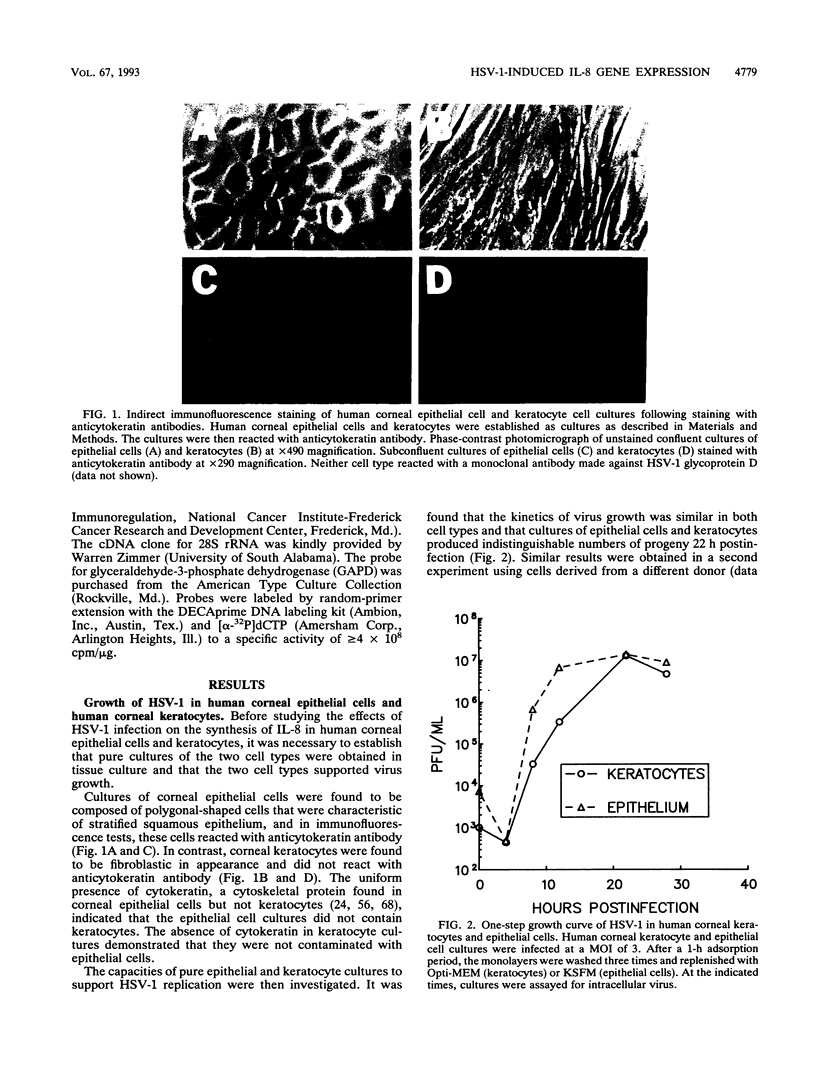
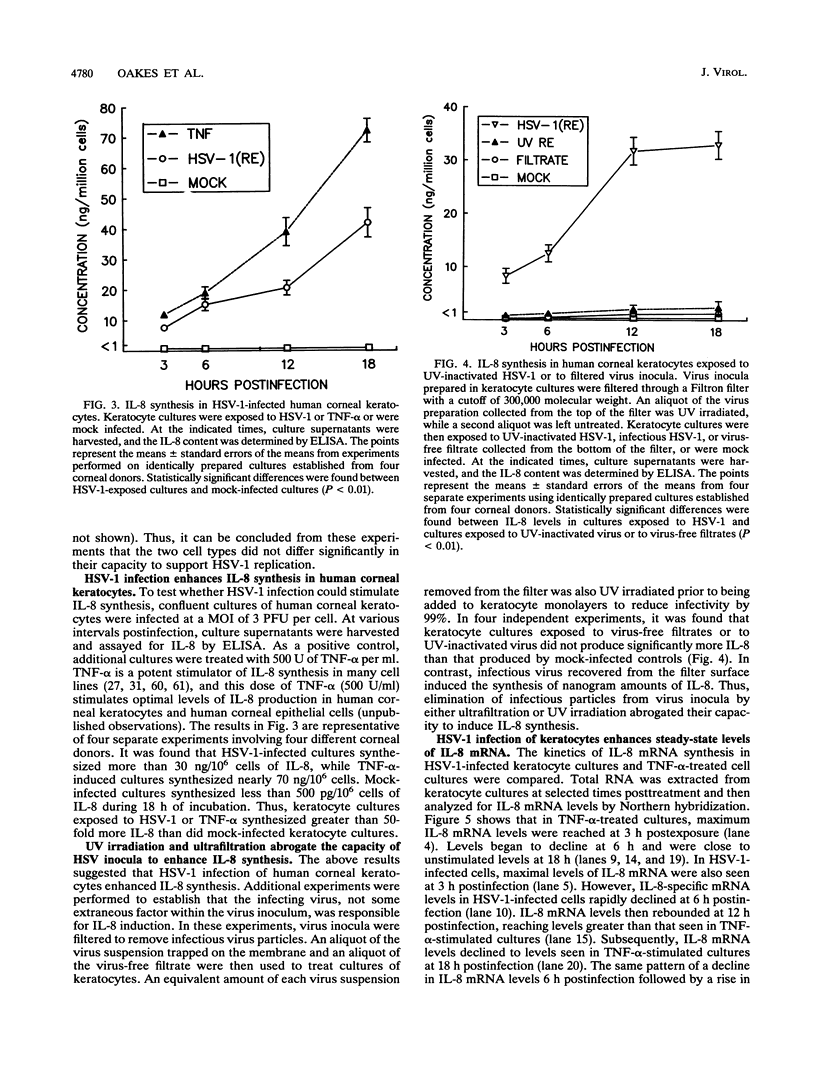
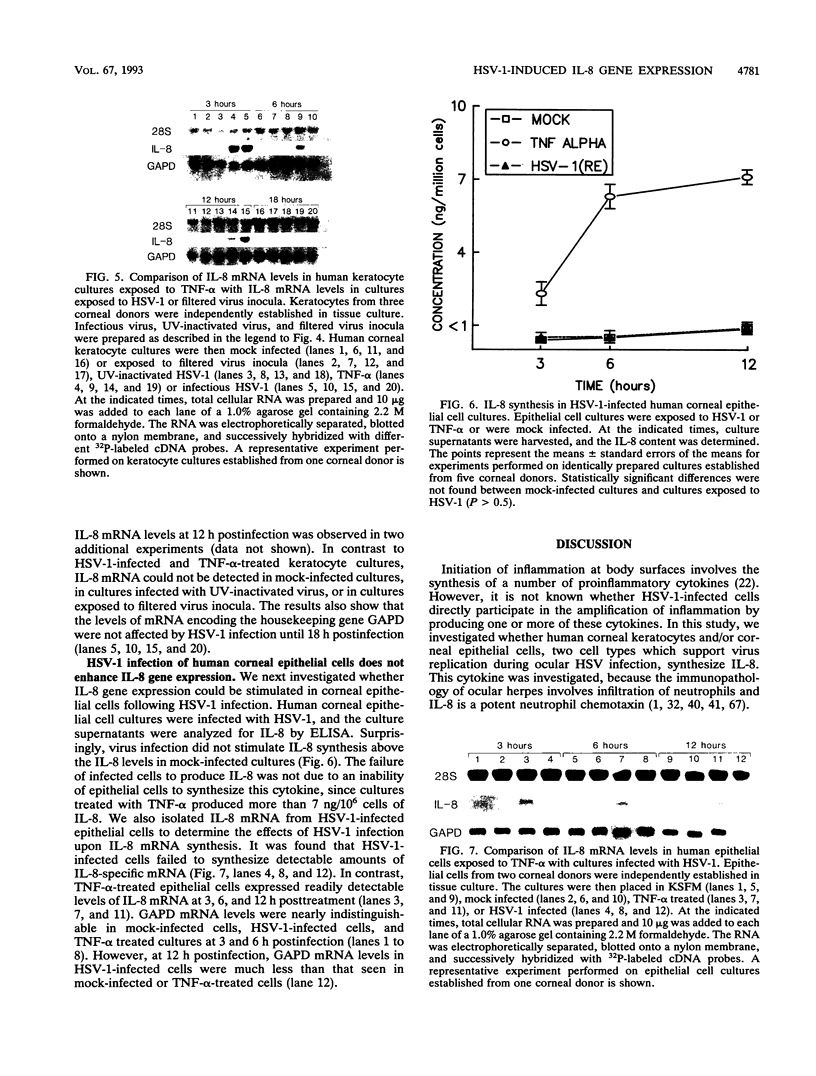
Abstract
Interleukin-8 (IL-8) is a proinflammatory cytokine released at sites of tissue damage by various cell types. One important function of IL-8 is to recruit neutrophils into sites of inflammation and to activate their biological activity. Stromal keratitis induced by herpes simplex virus type 1 (HSV-1) is characterized by an initial infiltration of neutrophils. This study was carried out to determine whether cells resident in the cornea synthesize IL-8 after virus infection. Pure cultures of epithelial cells and keratocytes established from human corneas were infected with HSV-1, and the medium overlying the cells was subsequently assayed for IL-8 by an enzyme-linked immunosorbent assay. Cytokine mRNA levels in cell lysates were monitored by Northern (RNA) blot analysis. It was found that virus infection of keratocyte cultures led to the synthesis of IL-8-specific mRNA with more than 30 ng of IL-8 made per 10(6) cells. Neither UV-inactivated virus nor virus-free filtrates collected from HSV-1-infected keratocytes could induce IL-8 protein or mRNA, suggesting that viral gene expression was needed for induction of IL-8 gene expression. Unlike keratocytes, HSV-1-infected epithelial cells failed to synthesize IL-8 protein or mRNA. However, these cells readily produced both molecules following tumor necrosis factor alpha stimulation. HSV-1 had similar titers in both cell types. Thus, the failure to induce IL-8 synthesis was not due to an inability of the virus to replicate in epithelial cells. The capacity of HSV-1-infected corneal keratocytes to synthesize IL-8 suggests that these cells can contribute to the induction of the acute inflammatory response seen in herpes stromal keratitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. N., Jones M. L., Mitra R. S., Crockett-Torabe E., Fantone J. C., Kunkel S. L., Warren J. S., Dixit V. M., Nickoloff B. J. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am J Pathol. 1991 Oct;139(4):869–876. [PMC free article] [PubMed] [Google Scholar]
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C. R., Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976 Sep-Oct;21(2):121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- Ebato B., Friend J., Thoft R. A. Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1450–1456. [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Pavilack M. A., Elner S. G., Remick D. G., Danforth J. M., Kunkel S. L. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am J Pathol. 1991 Nov;139(5):977–988. [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 1985 Aug;4(8):1973–1980. doi: 10.1002/j.1460-2075.1985.tb03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., Everett R. D. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J Gen Virol. 1990 Dec;71(Pt 12):2961–2967. doi: 10.1099/0022-1317-71-12-2961. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., McMenamin M. M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J Gen Virol. 1984 Jul;65(Pt 7):1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Wagner E. K. A bi-functional reporter plasmid for the simultaneous transient expression assay of two herpes simplex virus promoters. Virus Genes. 1987 Nov;1(1):61–71. doi: 10.1007/BF00125686. [DOI] [PubMed] [Google Scholar]
- Gosselin J., Flamand L., D'Addario M., Hiscott J., Stefanescu I., Ablashi D. V., Gallo R. C., Menezes J. Modulatory effects of Epstein-Barr, herpes simplex, and human herpes-6 viral infections and coinfections on cytokine synthesis. A comparative study. J Immunol. 1992 Jul 1;149(1):181–187. [PubMed] [Google Scholar]
- Jang K. L., Latchman D. S. The herpes simplex virus immediate-early protein ICP27 stimulates the transcription of cellular Alu repeated sequences by increasing the activity of transcription factor TFIIIC. Biochem J. 1992 Jun 15;284(Pt 3):667–673. doi: 10.1042/bj2840667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Latchman D. S. Induction and repression of cellular gene transcription during herpes simplex virus infection are mediated by different viral immediate-early gene products. Eur J Biochem. 1988 Jun 1;174(2):443–449. doi: 10.1111/j.1432-1033.1988.tb14118.x. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Latchman D. S. The herpes simplex virus type 1 immediate-early protein ICP4 specifically induces increased transcription of the human ubiquitin B gene without affecting the ubiquitin A and C genes. Virology. 1988 Sep;166(1):258–261. doi: 10.1016/0042-6822(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Key N. S., Vercellotti G. M., Winkelmann J. C., Moldow C. F., Goodman J. L., Esmon N. L., Esmon C. T., Jacob H. S. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw P. T., Schultz G. S., MacKay S. L., Chegini N., Rotatori D. S., Adams J. L., Shimizu R. W. Detection of transforming growth factor-alpha messenger RNA and protein in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992 Nov;33(12):3302–3306. [PubMed] [Google Scholar]
- Kiritoshi A., SundarRaj N., Thoft R. A. Differentiation in cultured limbal epithelium as defined by keratin expression. Invest Ophthalmol Vis Sci. 1991 Nov;32(12):3073–3077. [PubMed] [Google Scholar]
- Krikorian C. R., Read G. S. In vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J Virol. 1991 Jan;65(1):112–122. doi: 10.1128/jvi.65.1.112-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest. 1990 Dec;86(6):1783–1789. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus M. A., Stock E. L., Jones J. C. Expression of the 55-kD/64-kD corneal keratins in ocular surface epithelium. Invest Ophthalmol Vis Sci. 1990 Mar 1;31(3):448–456. [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Lausch R. N., Kleinschradt W. R., Monteiro C., Kayes S. G., Oakes J. E. Resolution of HSV corneal infection in the absence of delayed-type hypersensitivity. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1509–1515. [PubMed] [Google Scholar]
- Lausch R. N., Oakes J. E., Metcalf J. F., Scimeca J. M., Smith L. A., Robertson S. M. Quantitation of purified monoclonal antibody needed to prevent HSV-1 induced stromal keratitis in mice. Curr Eye Res. 1989 May;8(5):499–506. doi: 10.3109/02713688909000030. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Crabtree G. R. HNF-1, a member of a novel class of dimerizing homeodomain proteins. J Biol Chem. 1991 Jan 15;266(2):677–680. [PubMed] [Google Scholar]
- Metcalf J. F., Hamilton D. S., Reichert R. W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979 Dec;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Kaufman H. E. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976 Dec;82(6):827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Meyers-Elliott R. H., Chitjian P. A. Immunopathogenesis of corneal inflammation in herpes simplex virus stromal keratitis: role of the polymorphonuclear leukocyte. Invest Ophthalmol Vis Sci. 1981 Jun;20(6):784–798. [PubMed] [Google Scholar]
- Meyers R. L., Chitjian P. A. Immunology of herpesvirus infection: immunity to herpes simplex virus in eye infections. Surv Ophthalmol. 1976 Sep-Oct;21(2):194–204. doi: 10.1016/0039-6257(76)90100-4. [DOI] [PubMed] [Google Scholar]
- Meyers R. L., Pettit T. H. Chemotaxis of polymorphonuclear leukocytes in corneal inflammation: tissue injury in herpes simplex virus infection. Invest Ophthalmol. 1974 Mar;13(3):187–197. [PubMed] [Google Scholar]
- Meyers R. L., Pettit T. H. The pathogenesis of corneal inflammation due to Herpes simplex virus. I. Corneal hypersensitivity in the rabbit. J Immunol. 1973 Oct;111(4):1031–1042. [PubMed] [Google Scholar]
- Mielke V., Bauman J. G., Sticherling M., Ibs T., Zomershoe A. G., Seligmann K., Henneicke H. H., Schröder J. M., Sterry W., Christophers E. Detection of neutrophil-activating peptide NAP/IL-8 and NAP/IL-8 mRNA in human recombinant IL-1 alpha- and human recombinant tumor necrosis factor-alpha-stimulated human dermal fibroblasts. An immunocytochemical and fluorescent in situ hybridization study. J Immunol. 1990 Jan 1;144(1):153–161. [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Offord E. A., Leake R. E., Macnab J. C. Stimulation of estrogen receptor mRNA levels in MCF-7 cells by herpes simplex virus infection. J Virol. 1989 May;63(5):2388–2391. doi: 10.1128/jvi.63.5.2388-2391.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Oroskar A. A., Read G. S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989 May;63(5):1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavassiliou A. G., Silverstein S. J. Interaction of cell and virus proteins with DNA sequences encompassing the promoter/regulatory and leader regions of the herpes simplex virus thymidine kinase gene. J Biol Chem. 1990 Jun 5;265(16):9402–9412. [PubMed] [Google Scholar]
- Patel R., Chan W. L., Kemp L. M., La Thangue N. B., Latchman D. S. Isolation of cDNA clones derived from a cellular gene transcriptionally induced by herpes simplex virus. Nucleic Acids Res. 1986 Jul 25;14(14):5629–5640. doi: 10.1093/nar/14.14.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B., Abravaya K., Morimoto R. I. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. J Virol. 1991 Nov;65(11):5680–5692. doi: 10.1128/jvi.65.11.5680-5692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong B. L., Libermann T. A., Kogawa K., Ghosh S., Cao L. X., Pavan-Langston D., Dunkel E. C. HSV-1-inducible proteins bind to NF-kappa B-like sites in the HSV-1 genome. Virology. 1992 Aug;189(2):750–756. doi: 10.1016/0042-6822(92)90599-k. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Nasisse M. P., Larsen H. S., Rouse B. T. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):938–944. [PubMed] [Google Scholar]
- Schermer A., Galvin S., Sun T. T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986 Jul;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Levin M., Crumpacker C. S., Gilchrest B. A. Virus replication and induction of interferon in human epidermal keratinocytes following infection with herpes simplex virus. J Invest Dermatol. 1984 Jan;82(1):94–96. doi: 10.1111/1523-1747.ep12259193. [DOI] [PubMed] [Google Scholar]
- Sica A., Matsushima K., Van Damme J., Wang J. M., Polentarutti N., Dejana E., Colotta F., Mantovani A. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology. 1990 Apr;69(4):548–553. [PMC free article] [PubMed] [Google Scholar]
- Smibert C. A., Johnson D. C., Smiley J. R. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J Gen Virol. 1992 Feb;73(Pt 2):467–470. doi: 10.1099/0022-1317-73-2-467. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Su Y. H., Oakes J. E., Lausch R. N. Ocular avirulence of a herpes simplex virus type 1 strain is associated with heightened sensitivity to alpha/beta interferon. J Virol. 1990 May;64(5):2187–2192. doi: 10.1128/jvi.64.5.2187-2192.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Van Horn D. L., Edelhauser H. E., Schultz R. O. Experimental herpes simplex keratitis. Early alterations of corneal epithelium and stroma. Arch Ophthalmol. 1970 Jul;84(1):67–75. doi: 10.1001/archopht.1970.00990040069018. [DOI] [PubMed] [Google Scholar]
- Walz A., Burgener R., Car B., Baggiolini M., Kunkel S. L., Strieter R. M. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991 Dec 1;174(6):1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Komarov P., Sies H., de Groot H. Inhibition of superoxide and nitric oxide release and protection from reoxygenation injury by Ebselen in rat Kupffer cells. Hepatology. 1992 Jun;15(6):1112–1116. doi: 10.1002/hep.1840150623. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Wiley L., SundarRaj N., Sun T. T., Thoft R. A. Regional heterogeneity in human corneal and limbal epithelia: an immunohistochemical evaluation. Invest Ophthalmol Vis Sci. 1991 Mar;32(3):594–602. [PubMed] [Google Scholar]