Abstract
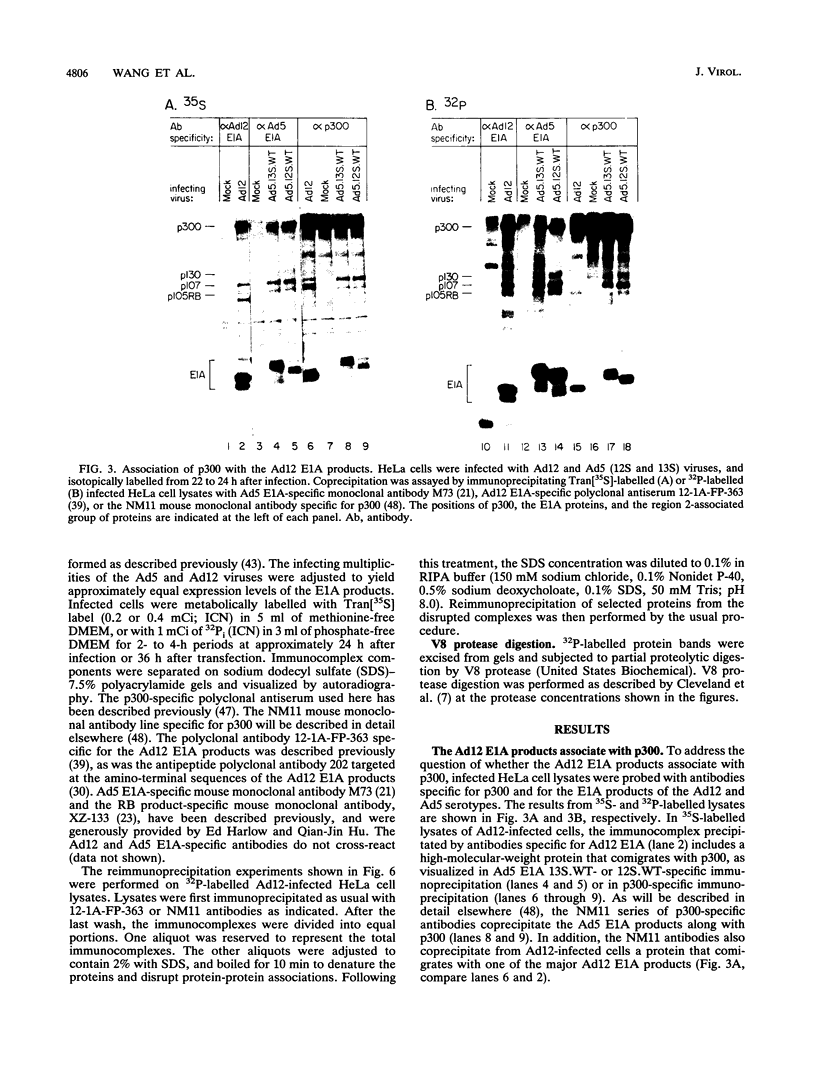
The cell growth-regulating properties of the adenovirus type 5 (Ad5) E1A oncogene correlate closely with the binding of the E1A products to specific cellular proteins. These proteins include the products of the retinoblastoma tumor susceptibility gene and a 300-kDa product, p300. pRB binds to E1A sequences that are highly conserved among the E1A products of various serotypes, while p300 binding requires sequences in the E1A amino terminus, a region that is not highly conserved. To help evaluate the roles of the E1A-associated proteins in cell growth control, we have compared the p300-binding abilities of the E1A products of Ad5 and of the more oncogenic Ad12 serotype. We show here that despite encoding a sequence that varies somewhat from the p300-binding sequences of Ad5 E1A, the Ad12 E1A products associate with p300 with an affinity similar to that of the Ad5 E1A products. Both the 12S and 13S splice products of Ad12 E1A, like those of Ad5 E1A, encode proteins able to associate with p300. Interestingly, though, both also give rise to prominent forms that are amino terminally modified and unable to associate with p300. This modification, at least in the 13S product, does not appear to diminish the affinity of this product for the retinoblastoma protein.
Full text
PDF

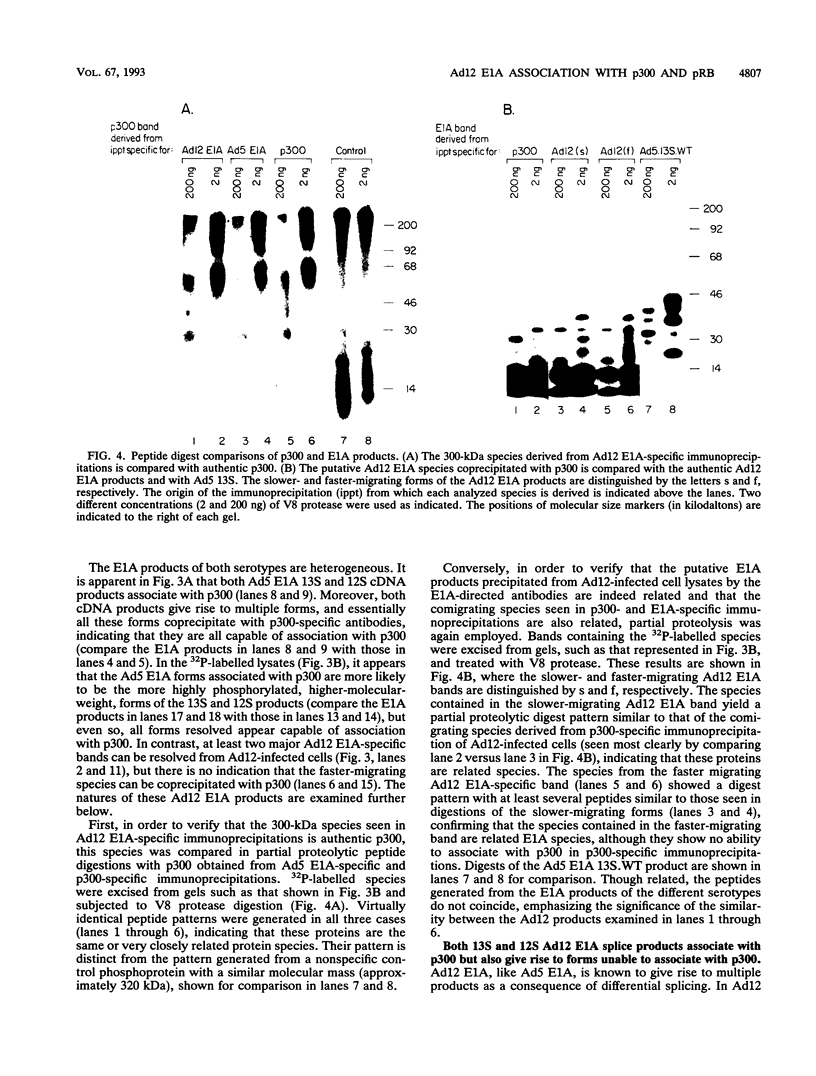

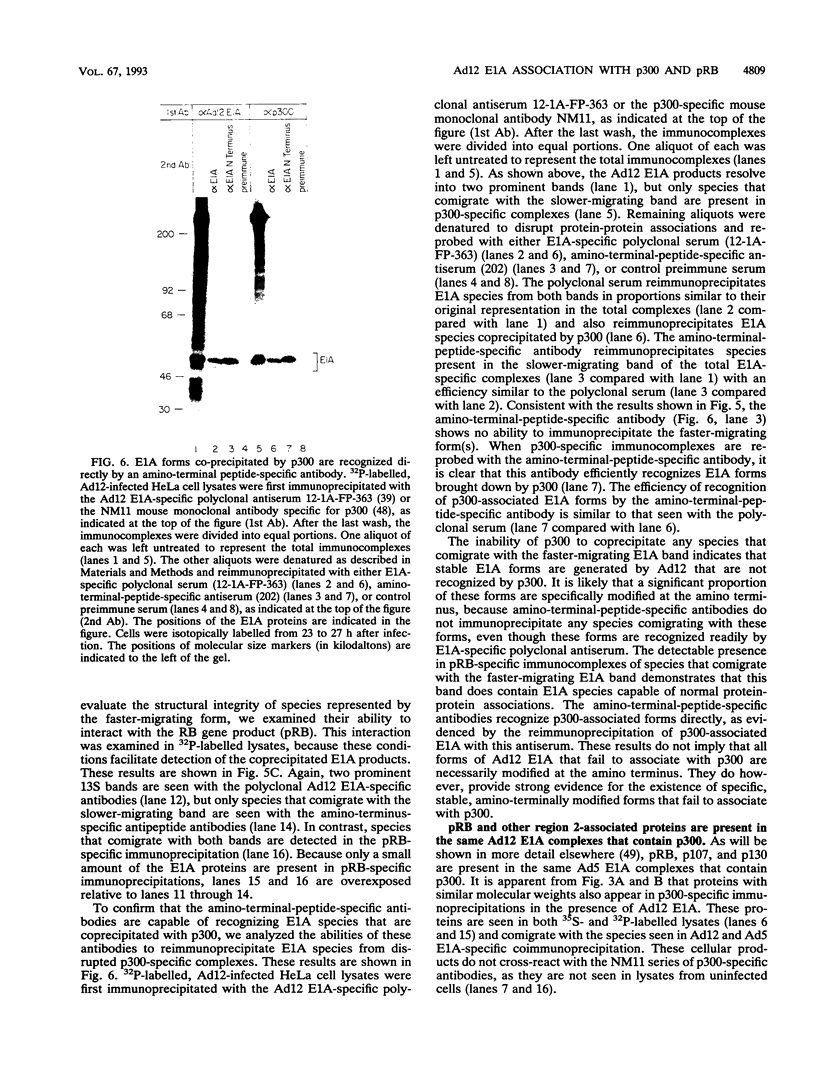




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. E., Lobo S., Yaciuk P., Wang H. G., Moran E. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene. 1993 Jun;8(6):1639–1647. [PubMed] [Google Scholar]
- Allard A., Wadell G. Physical organization of the enteric adenovirus type 41 early region 1A. Virology. 1988 May;164(1):220–229. doi: 10.1016/0042-6822(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Blair G. E. Expression and interactions of human adenovirus oncoproteins. Biochem J. 1991 Apr 15;275(Pt 2):281–299. doi: 10.1042/bj2750281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulukos K. E., Ziff E. B. Adenovirus 5 E1A proteins disrupt the neuronal phenotype and growth factor responsiveness of PC12 cells by a conserved region 1-dependent mechanism. Oncogene. 1993 Feb;8(2):237–248. [PubMed] [Google Scholar]
- Brockmann D., Tries B., Esche H. Isolation and characterization of novel adenovirus type 12 E1A mRNAs by cDNA PCR technique. Virology. 1990 Dec;179(2):585–590. doi: 10.1016/0042-6822(90)90125-b. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cobrinik D., Dowdy S. F., Hinds P. W., Mittnacht S., Weinberg R. A. The retinoblastoma protein and the regulation of cell cycling. Trends Biochem Sci. 1992 Aug;17(8):312–315. doi: 10.1016/0968-0004(92)90443-d. [DOI] [PubMed] [Google Scholar]
- Dyson N., Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. J., Ricciardi R. P. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology. 1988 Jul;165(1):303–305. doi: 10.1016/0042-6822(88)90689-7. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Paraskeva C. A study to determine the reasons for differences in the tumorigenicity of rat cell lines transformed by adenovirus 2 and adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):703–713. doi: 10.1101/sqb.1980.044.01.075. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. J., Bautista C., Edwards G. M., Defeo-Jones D., Jones R. E., Harlow E. Antibodies specific for the human retinoblastoma protein identify a family of related polypeptides. Mol Cell Biol. 1991 Nov;11(11):5792–5799. doi: 10.1128/mcb.11.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino M., Ohashi Y., Emoto T., Sawada Y., Fujinaga K. Characterization of adenovirus type 40 E1 region. Virology. 1988 Jul;165(1):95–102. doi: 10.1016/0042-6822(88)90662-9. [DOI] [PubMed] [Google Scholar]
- Jelinek T., Graham F. L. Recombinant human adenoviruses containing hybrid adenovirus type 5 (Ad5)/Ad12 E1A genes: characterization of hybrid E1A proteins and analysis of transforming activity and host range. J Virol. 1992 Jul;66(7):4117–4125. doi: 10.1128/jvi.66.7.4117-4125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen A. G., Bos J. L., van der Eb A. J. The first exon of region E1a genes of adenoviruses 5 and 12 encodes a separate functional protein domain. EMBO J. 1984 Dec 1;3(12):2923–2927. doi: 10.1002/j.1460-2075.1984.tb02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S., Ozawa K., Kondoh S., Soeda E., Israel A., Shiroki K., Fujinaga K., Itakura K., Gachelin G., Yokoyama K. Identification of sequences responsible for positive and negative regulation by E1A in the promoter of H-2Kbm1 class I MHC gene. EMBO J. 1990 Jan;9(1):127–135. doi: 10.1002/j.1460-2075.1990.tb08088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus V. B., Moran E., Nevins J. R. Promoter-specific trans-activation by the adenovirus E1A12S product involves separate E1A domains. Mol Cell Biol. 1992 Oct;12(10):4391–4399. doi: 10.1128/mcb.12.10.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucher L. A., Brackmann K. H., Symington J. S., Green M. Posttranslational modification at the N terminus of the human adenovirus type 12 E1A 235R tumor antigen. J Virol. 1986 May;58(2):592–599. doi: 10.1128/jvi.58.2.592-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran B., Zerler B. Interactions between cell growth-regulating domains in the products of the adenovirus E1A oncogene. Mol Cell Biol. 1988 Apr;8(4):1756–1764. doi: 10.1128/mcb.8.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E. DNA tumor virus transforming proteins and the cell cycle. Curr Opin Genet Dev. 1993 Feb;3(1):63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- Moran E., Grodzicker T., Roberts R. J., Mathews M. B., Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. doi: 10.1128/jvi.57.3.765-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- PEREIRA H. G., HUEBNER R. J., GINSBERG H. S., VAN DER VEEN J. A SHORT DESCRIPTION OF THE ADENOVIRUS GROUP. Virology. 1963 Aug;20:613–620. doi: 10.1016/0042-6822(63)90286-1. [DOI] [PubMed] [Google Scholar]
- Rikitake Y., Moran E. DNA-binding properties of the E1A-associated 300-kilodalton protein. Mol Cell Biol. 1992 Jun;12(6):2826–2836. doi: 10.1128/mcb.12.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Yee S. P., Otis J., Graham F. L., Branton P. E. Characterization of human adenovirus type 5 early region 1A polypeptides using antitumor sera and an antiserum specific for the carboxy terminus. Virology. 1983 Jun;127(2):253–271. doi: 10.1016/0042-6822(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Scott M. O., Kimelman D., Norris D., Ricciardi R. P. Production of a monospecific antiserum against the early region 1A proteins of adenovirus 12 and adenovirus 5 by an adenovirus 12 early region 1A-beta-galactosidase fusion protein antigen expressed in bacteria. J Virol. 1984 Jun;50(3):895–903. doi: 10.1128/jvi.50.3.895-903.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987 Jul;6(7):2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga O., Yaegashi T., Lowe J., Dobbs L., Padmanabhan R. Sequence analysis in the E1 region of adenovirus type 4 DNA. Virology. 1986 Dec;155(2):418–433. doi: 10.1016/0042-6822(86)90204-7. [DOI] [PubMed] [Google Scholar]
- Ulfendahl P. J., Linder S., Kreivi J. P., Nordqvist K., Sevensson C., Hultberg H., Akusjärvi G. A novel adenovirus-2 E1A mRNA encoding a protein with transcription activation properties. EMBO J. 1987 Jul;6(7):2037–2044. doi: 10.1002/j.1460-2075.1987.tb02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. G., Draetta G., Moran E. E1A induces phosphorylation of the retinoblastoma protein independently of direct physical association between the E1A and retinoblastoma products. Mol Cell Biol. 1991 Aug;11(8):4253–4265. doi: 10.1128/mcb.11.8.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. G., Rikitake Y., Carter M. C., Yaciuk P., Abraham S. E., Zerler B., Moran E. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and to the control of cell growth. J Virol. 1993 Jan;67(1):476–488. doi: 10.1128/jvi.67.1.476-488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yaciuk P., Moran E. Analysis with specific polyclonal antiserum indicates that the E1A-associated 300-kDa product is a stable nuclear phosphoprotein that undergoes cell cycle phase-specific modification. Mol Cell Biol. 1991 Nov;11(11):5389–5397. doi: 10.1128/mcb.11.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]