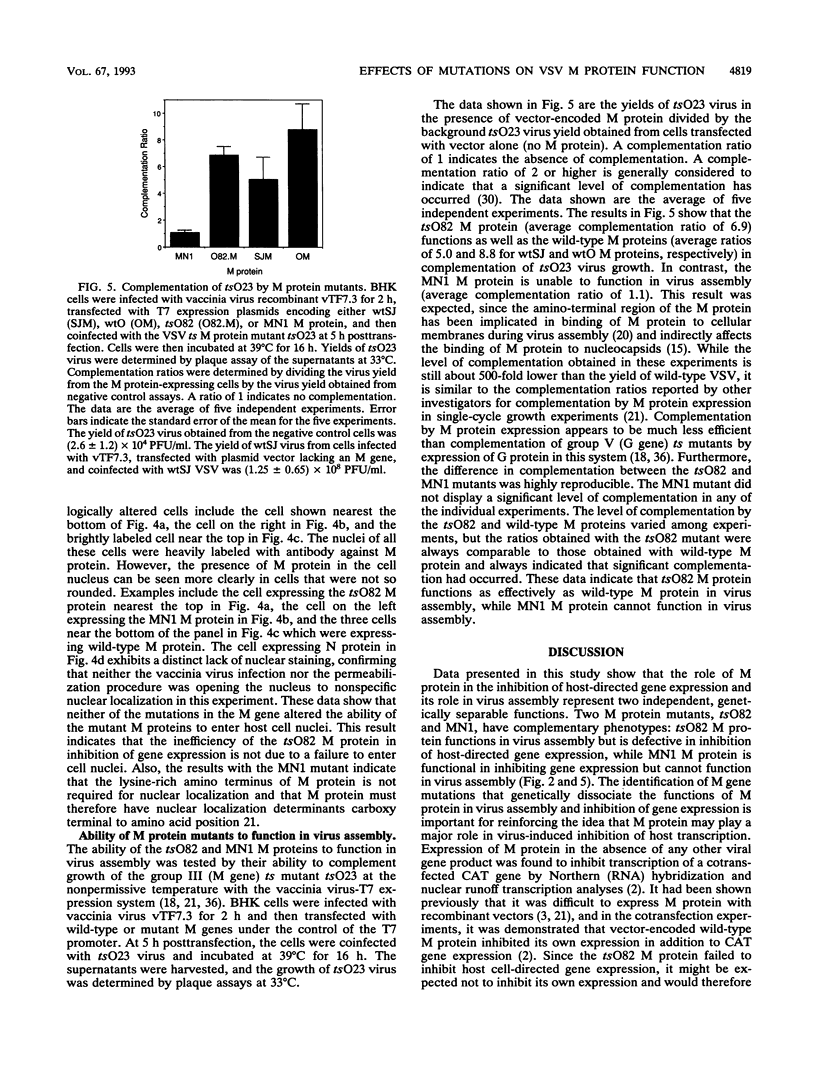
Abstract
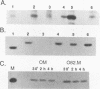
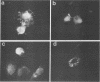
Recently, the vesicular stomatitis virus matrix (M) protein has been shown to be capable of inhibition of host cell-directed transcription in the absence of other viral components (B. L. Black and D. S. Lyles, J. Virol. 66:4058-4064, 1992). M protein is a major structural protein that is known to play a critical role in virus assembly by binding the helical ribonucleoprotein core of the virus to the cytoplasmic surface of the cell plasma membrane during budding. In this study, two M protein mutants were tested to determine whether the inhibition of host transcription by M protein is an indirect effect of its function in virus assembly or whether it represents an independent function of M protein. The mutant M protein of the conditionally temperature-sensitive (ts) vesicular stomatitis virus mutant, tsO82, was found to be defective in its ability to inhibit host-directed gene expression, as shown by its inability to inhibit expression of a cotransfected target gene encoding chloramphenicol acetyltransferase. The ability of the tsO82 M protein to function in virus assembly was similar to that of wild-type M protein, as shown by its ability to complement the group III ts M protein mutant, tsO23. Another mutant, MN1, which lacks amino acids 4 to 21 of M protein demonstrated that the abilities of M protein to inhibit chloramphenicol acetyltransferase gene expression and to localize to the nucleus were unaffected by deletion of this lysine-rich amino-terminal region but that the ability to function in virus assembly was ablated. Thus, the two M protein mutants examined in this study exhibited complementary phenotypes: tsO82 M protein functioned in virus assembly but was defective in inhibition of host-directed gene expression, while MN1 M protein functioned in inhibiting gene expression but was unable to function in virus assembly. These data demonstrate that the role of M protein in inhibition of host transcription can be separated genetically from its role in virus assembly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxt B., Bablanian R. Mechansims of vesicular stomatitis virus-induced cytopathic effects. II. Inhibition of macromolecular synthesis induced by infectious and defective-interfering particles. Virology. 1976 Jul 15;72(2):383–392. doi: 10.1016/0042-6822(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Black B. L., Lyles D. S. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J Virol. 1992 Jul;66(7):4058–4064. doi: 10.1128/jvi.66.7.4058-4064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel D., Harmison G. G., Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990 Apr;64(4):1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman M. R., Lyles D. S., Parce J. W. Possible mechanisms by which the H-2Kbm3 mutation may decrease cytotoxic T-lymphocyte recognition of vesicular stomatitis virus nucleoprotein antigen. J Virol. 1987 Jun;61(6):1992–1998. doi: 10.1128/jvi.61.6.1992-1998.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J., McElhone P., Brokaw J., Anker R., Pollok B. A. Co-expression of full-length and truncated Ig mu-chains in human B lymphocytes results from alternative splicing of a single primary RNA transcript. J Immunol. 1991 Jun 15;146(12):4344–4351. [PubMed] [Google Scholar]
- Coulon P., Deutsch V., Lafay F., Martinet-Edelist C., Wyers F., Herman R. C., Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J Gen Virol. 1990 Apr;71(Pt 4):991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Baird S., Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986 Apr 15;150(1):231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983 Oct;48(1):88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Inhibition of DNA-dependent transcription by the leader RNA of vesicular stomatitis virus: role of specific nucleotide sequences and cell protein binding. Mol Cell Biol. 1985 Oct;5(10):2502–2513. doi: 10.1128/mcb.5.10.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Kaptur P. E., Rhodes R. B., Lyles D. S. Sequences of the vesicular stomatitis virus matrix protein involved in binding to nucleocapsids. J Virol. 1991 Mar;65(3):1057–1065. doi: 10.1128/jvi.65.3.1057-1065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz E. J., Pattnaik A. K., Ball L. A. Complementation of a vesicular stomatitis virus glycoprotein G mutant with wild-type protein expressed from either a bovine papilloma virus or a vaccinia virus vector system. Virology. 1990 Oct;178(2):373–383. doi: 10.1016/0042-6822(90)90334-n. [DOI] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lenard J., Vanderoef R. Localization of the membrane-associated region of vesicular stomatitis virus M protein at the N terminus, using the hydrophobic, photoreactive probe 125I-TID. J Virol. 1990 Jul;64(7):3486–3491. doi: 10.1128/jvi.64.7.3486-3491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Expression of the M gene of vesicular stomatitis virus cloned in various vaccinia virus vectors. J Virol. 1988 Mar;62(3):776–782. doi: 10.1128/jvi.62.3.776-782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Morita K., Vanderoef R., Lenard J. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J Virol. 1987 Feb;61(2):256–263. doi: 10.1128/jvi.61.2.256-263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot M. K., Schnitzlein W. M., Reichmann M. E. The requirement of protein synthesis for VSV inhibition of host cell RNA synthesis. Virology. 1985 Jan 15;140(1):91–101. doi: 10.1016/0042-6822(85)90448-9. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J. B., Pal R., Wagner R. R. Antigenicity, function, and conformation of synthetic oligopeptides corresponding to amino-terminal sequences of wild-type and mutant matrix proteins of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2569–2577. doi: 10.1128/jvi.62.8.2569-2577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt M. A., Chong L., Rose J. K. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989 Sep;63(9):3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi Y., Mitsui H., Amano M. Effect of U.v.-irradiated vesicular stomatitis virus on nucleic acid synthesis in chick embryo cells. J Gen Virol. 1970 Sep;8(3):165–172. doi: 10.1099/0022-1317-8-3-165. [DOI] [PubMed] [Google Scholar]