Abstract
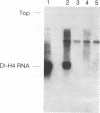
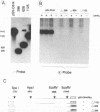
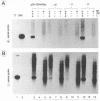
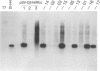
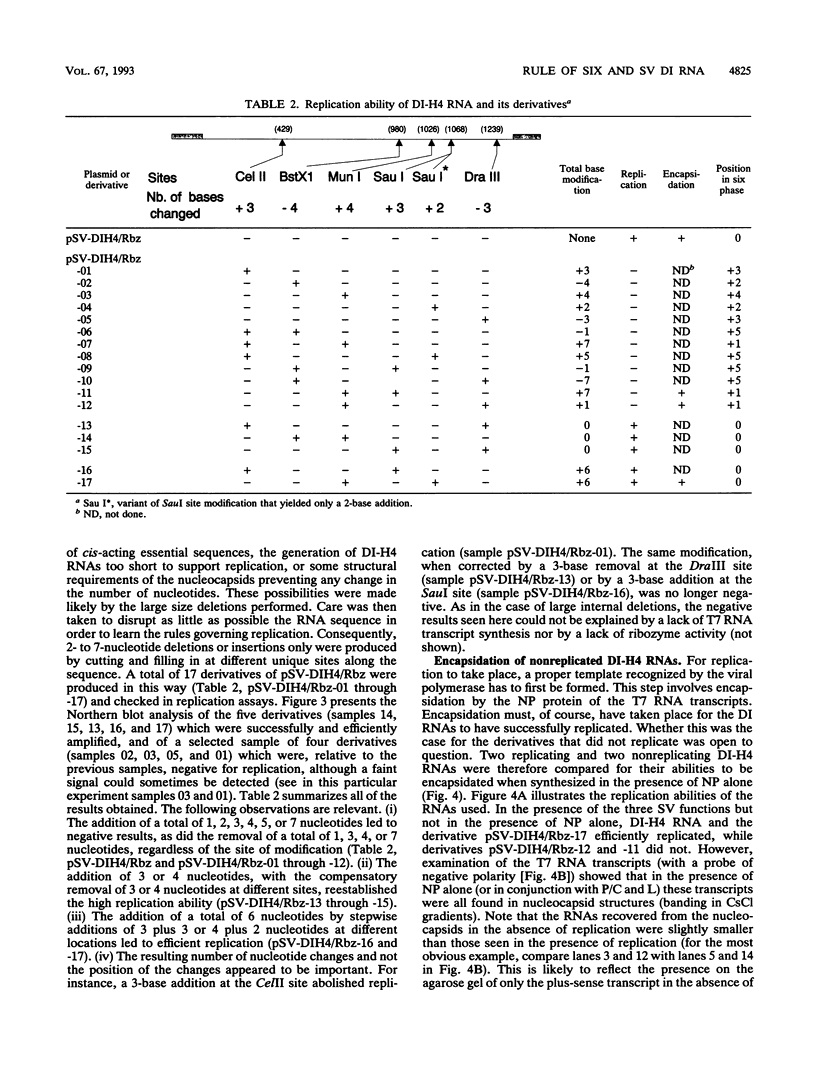
The addition of the hepatitis delta virus genomic ribozyme to the 3' end sequence of a Sendai virus defective interfering RNA (DI-H4) allowed the reproducible and efficient replication of this RNA by the viral functions expressed from cloned genes when the DI RNA was synthesized from plasmid. Limited nucleotide additions or deletions (+7 to -7 nucleotides) in the DI RNA sequence were then made at five different sites, and the different RNA derivatives were tested for their abilities to replicate. Efficient replication was observed only when the total nucleotide number was conserved, regardless of the modifications, or when the addition of a total of 6 nucleotides was made. The replicated RNAs were shown to be properly enveloped into virus particles. It is concluded that, to form a proper template for efficient replication, the Sendai virus RNA must contain a total number of nucleotides which is a multiple of 6. This was interpreted as the need for the nucleocapsid protein to contact exactly 6 nucleotides.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Kolakofsky D. Intracellular vesicular stomatitis virus leader RNAs are found in nucleocapsid structures. J Virol. 1981 Nov;40(2):568–576. doi: 10.1128/jvi.40.2.568-576.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calain P., Curran J., Kolakofsky D., Roux L. Molecular cloning of natural paramyxovirus copy-back defective interfering RNAs and their expression from DNA. Virology. 1992 Nov;191(1):62–71. doi: 10.1016/0042-6822(92)90166-m. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Stec D. S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Kolakofsky D. Rescue of a Sendai virus DI genome by other parainfluenza viruses: implications for genome replication. Virology. 1991 May;182(1):168–176. doi: 10.1016/0042-6822(91)90660-4. [DOI] [PubMed] [Google Scholar]
- Curran J., Boeck R., Kolakofsky D. The Sendai virus P gene expresses both an essential protein and an inhibitor of RNA synthesis by shuffling modules via mRNA editing. EMBO J. 1991 Oct;10(10):3079–3085. doi: 10.1002/j.1460-2075.1991.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman E. H., Wu S. S., Amrein M., Portner A., Murti G. The Sendai virus nucleocapsid exists in at least four different helical states. J Virol. 1989 May;63(5):2233–2243. doi: 10.1128/jvi.63.5.2233-2243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Gibbs A. J. Observations on the structure of the nucleocapsids of some paramyxoviruses. J Gen Virol. 1970 Jan;6(1):141–150. doi: 10.1099/0022-1317-6-1-141. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Scheid A., Choppin P. W. the relationship of conformational changes in the Sendai virus nucleocapsid to proteolytic cleavage of the NP polypeptide. Virology. 1981 Oct 30;114(2):555–562. doi: 10.1016/0042-6822(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The gene structure and replication of influenza virus. Annu Rev Biochem. 1983;52:467–506. doi: 10.1146/annurev.bi.52.070183.002343. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Li X., Palese P. Mutational analysis of the promoter required for influenza virus virion RNA synthesis. J Virol. 1992 Jul;66(7):4331–4338. doi: 10.1128/jvi.66.7.4331-4338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Mink M. A., Stec D. S., Collins P. L. Nucleotide sequences of the 3' leader and 5' trailer regions of human respiratory syncytial virus genomic RNA. Virology. 1991 Dec;185(2):615–624. doi: 10.1016/0042-6822(91)90532-g. [DOI] [PubMed] [Google Scholar]
- Mottet G., Roux L. Budding efficiency of Sendai virus nucleocapsids: influence of size and ends of the RNA. Virus Res. 1989 Oct;14(2):175–187. doi: 10.1016/0168-1702(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Park K. H., Huang T., Correia F. F., Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Ball L. A., LeGrone A. W., Wertz G. W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992 Jun 12;69(6):1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991 Apr 4;350(6317):434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. The self-cleaving domain from the genomic RNA of hepatitis delta virus: sequence requirements and the effects of denaturant. Nucleic Acids Res. 1990 Dec 11;18(23):6821–6827. doi: 10.1093/nar/18.23.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]