Abstract
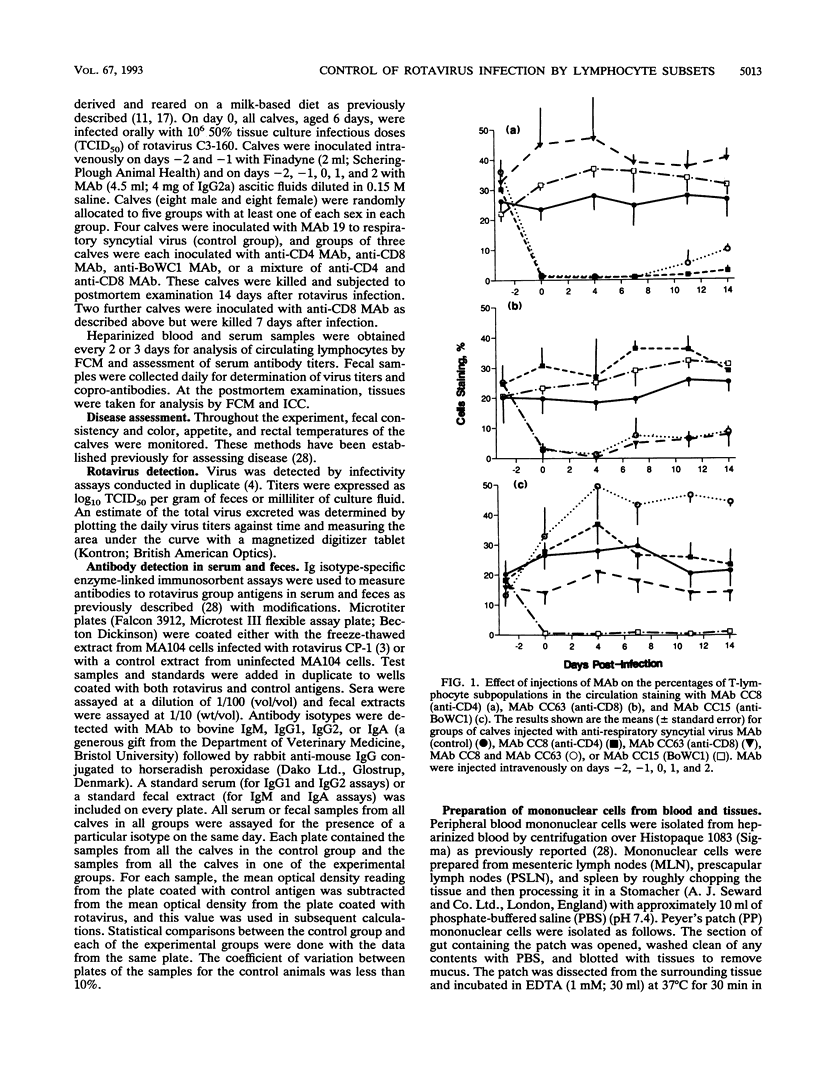
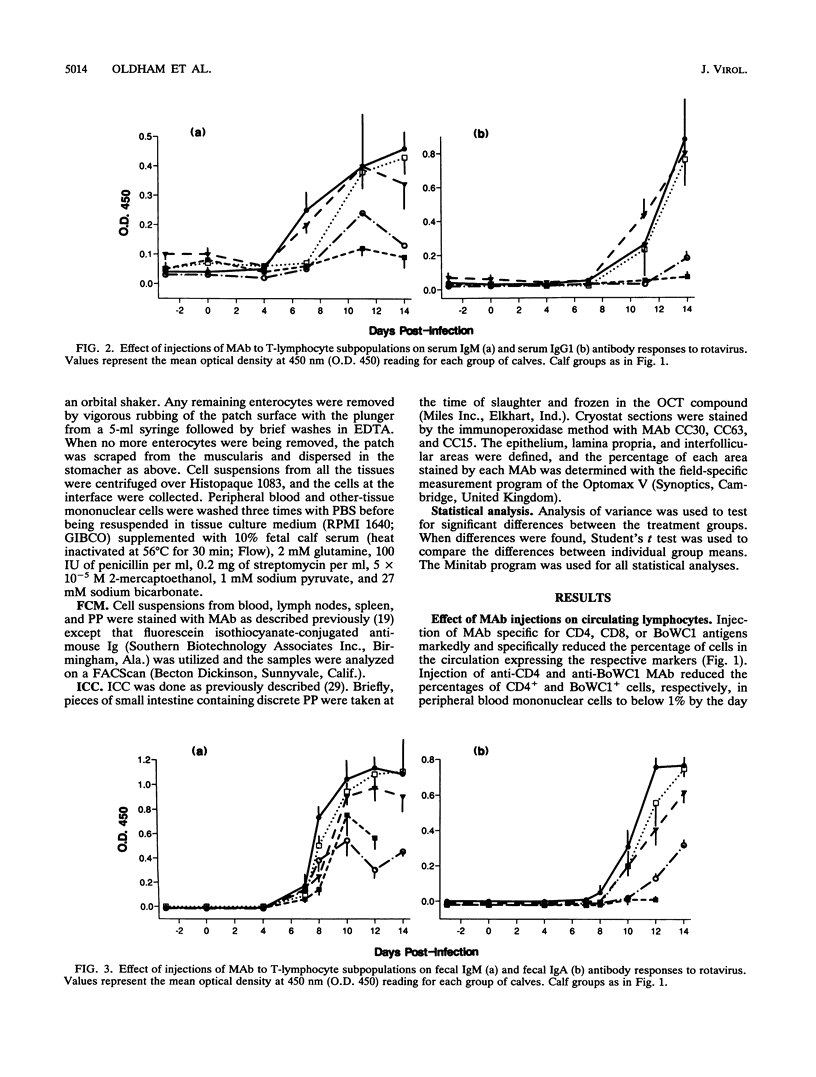
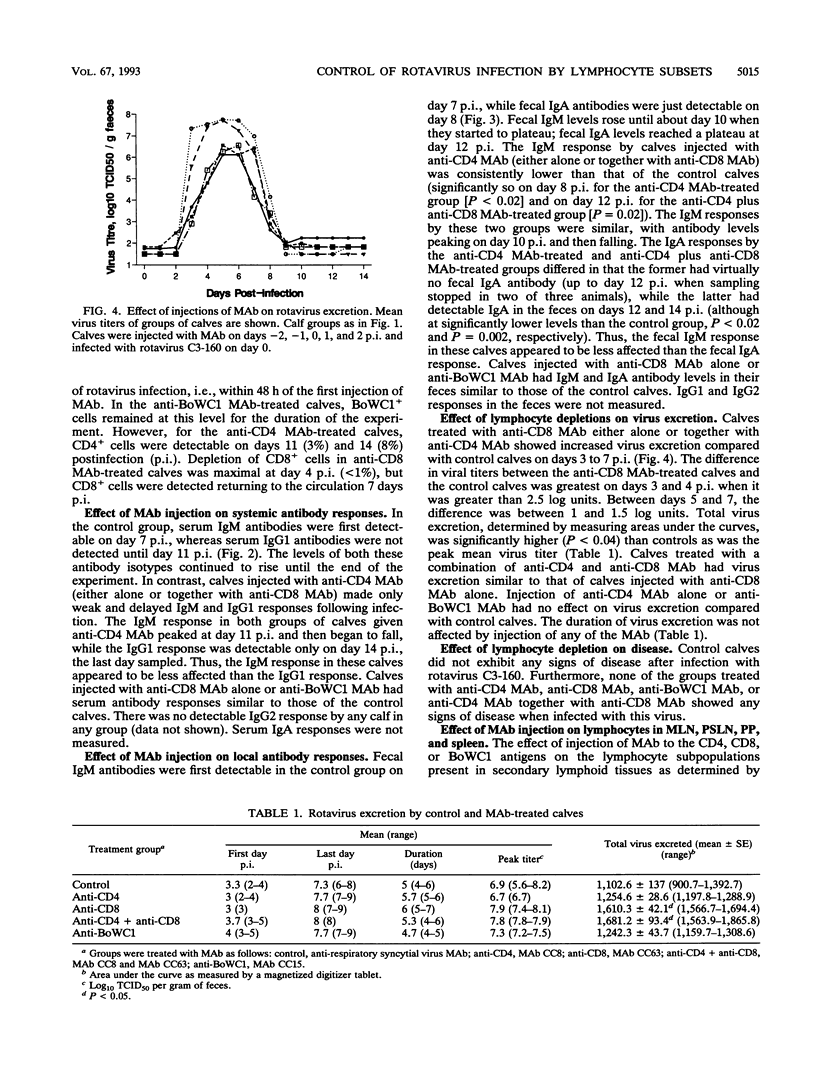
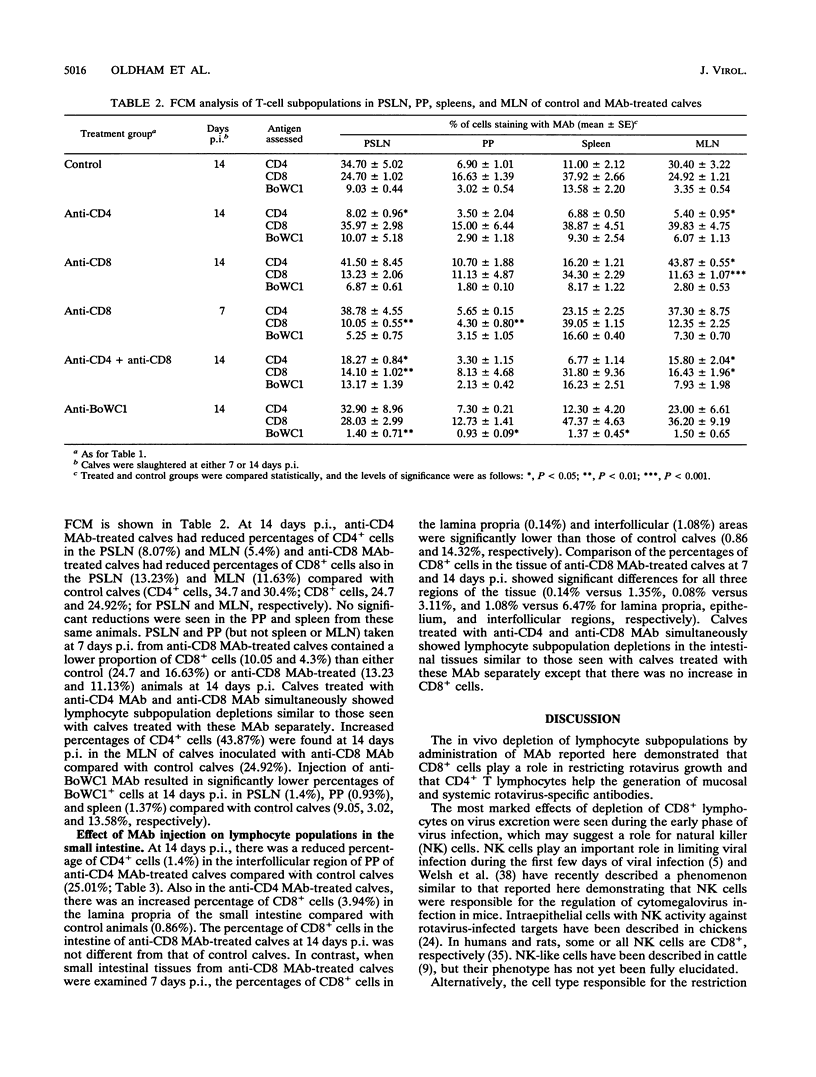
T-cell control of primary rotavirus infection and mucosal antibody responses to rotavirus was studied with monoclonal antibodies (MAb) to deplete gnotobiotic calves of CD4+, CD8+, BoWC1+, or both CD4+ and CD8+ lymphocytes prior to infection with rotavirus. Injection of these MAb produced specific reductions in circulating and tissue lymphocyte subpopulations. Following infection, control calves developed fecal immunoglobulin M (IgM) and IgA antibodies and serum IgM and IgG1 antibodies; there was no IgG2 antibody produced. Anti-CD4-treated calves had reduced fecal and serum antibody responses to rotavirus compared with control calves. The IgM response was less affected than the other isotypes. Calves concurrently injected with MAb to CD4 and CD8 had antibody responses similar to those of calves injected with anti-CD4 antibody alone. No effect on serum or fecal antibody levels was seen when MAb to CD8 or BoWC1 were injected alone. Virus excretion was significantly increased in calves depleted of CD8+ cells. Depletion of CD4+ cells or BoWC1+ cells had no effect on virus excretion. Calves depleted of both CD4+ and CD8+ cells excreted amounts of virus similar to those of calves depleted of CD8+ cells alone. Onset and duration of virus excretion were not affected by any of the MAb treatments. We conclude that a CD8+ cell population is involved in limiting primary rotavirus infection, while CD4+ or BoWC1+ (gamma/delta+ TcR) lymphocytes are not. Furthermore, CD4+ lymphocytes (but not CD8+ or BoWC1+ lymphocytes) were shown to be important in the generation of mucosal, as well as systemic, antibody responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensaid A., Hadam M. Individual antigens of cattle. Bovine CD4 (BoCD4). Vet Immunol Immunopathol. 1991 Jan;27(1-3):51–54. doi: 10.1016/0165-2427(91)90078-q. [DOI] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Brown J. F. Antigenic and pathogenic relationships of three bovine rotaviruses and a porcine rotavirus. J Gen Virol. 1984 Jul;65(Pt 7):1151–1158. doi: 10.1099/0022-1317-65-7-1151. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Pocock D. H. Variation in virulence of bovine rotaviruses. J Hyg (Lond) 1986 Apr;96(2):257–264. doi: 10.1017/s0022172400066031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation. 1986 May;41(5):634–639. doi: 10.1097/00007890-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Cook C. G., Splitter G. A. Comparison of bovine mononuclear cells with other species for cytolytic activity against virally-infected cells. Vet Immunol Immunopathol. 1989 Feb;20(3):239–261. doi: 10.1016/0165-2427(89)90004-4. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P. Enhancement of IgG production elicited in mice by treatment with anti-CD8 antibody. Eur J Immunol. 1991 Oct;21(10):2617–2620. doi: 10.1002/eji.1830211046. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Davies D. C., Hoare M. N. A simplified apparatus for the microbiological isolation of calves. Br Vet J. 1976 Nov-Dec;132(6):642–646. doi: 10.1016/s0007-1935(17)34542-6. [DOI] [PubMed] [Google Scholar]
- Dharakul T., Rott L., Greenberg H. B. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J Virol. 1990 Sep;64(9):4375–4382. doi: 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Eiden J., Lederman H. M., Vonderfecht S., Yolken R. T-cell-deficient mice display normal recovery from experimental rotavirus infection. J Virol. 1986 Feb;57(2):706–708. doi: 10.1128/jvi.57.2.706-708.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Owen R. L., Heyworth M. F. Modulation of lymphocyte subsets in Peyer's patches of mice treated with monoclonal antibody against helper T-cells. J Histochem Cytochem. 1988 Apr;36(4):417–423. doi: 10.1177/36.4.2964470. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992 Jul 1;149(1):175–180. [PubMed] [Google Scholar]
- Hoare M. N., Davies D. C., Dennis M. J. The derivation of gnotobiotic calves by a hysterotomy and slaughter technique. Br Vet J. 1976 Jul-Aug;132(4):369–373. doi: 10.1016/s0007-1935(17)34635-3. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R., Finch J. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur J Immunol. 1989 Apr;19(4):757–764. doi: 10.1002/eji.1830190428. [DOI] [PubMed] [Google Scholar]
- Kohl S., Harmon M. W., Tang J. P. Cytokine-stimulated human natural killer cytotoxicity: response to rotavirus-infected cells. Pediatr Res. 1983 Nov;17(11):868–872. doi: 10.1203/00006450-198311000-00006. [DOI] [PubMed] [Google Scholar]
- Leukocyte antigens in cattle, sheep and goats. Proceedings of the First International Workshop on Leukocyte Antigens in Cattle, Sheep and Goats. Hannover, Germany, 25 July 1989. Vet Immunol Immunopathol. 1991 Jan;27(1-3):1–275. [PubMed] [Google Scholar]
- London S. D., Rubin D. H., Cebra J. J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J Exp Med. 1987 Mar 1;165(3):830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHugh N. D., Sopp P. Individual antigens of cattle. Bovine CD8 (BoCD8). Vet Immunol Immunopathol. 1991 Jan;27(1-3):65–69. doi: 10.1016/0165-2427(91)90081-m. [DOI] [PubMed] [Google Scholar]
- Mega J., Bruce M. G., Beagley K. W., McGhee J. R., Taguchi T., Pitts A. M., McGhee M. L., Bucy R. P., Eldridge J. H., Mestecky J. Regulation of mucosal responses by CD4+ T lymphocytes: effects of anti-L3T4 treatment on the gastrointestinal immune system. Int Immunol. 1991 Aug;3(8):793–805. doi: 10.1093/intimm/3.8.793. [DOI] [PubMed] [Google Scholar]
- Myers T. J., Schat K. A. Natural killer cell activity of chicken intraepithelial leukocytes against rotavirus-infected target cells. Vet Immunol Immunopathol. 1990 Oct;26(2):157–170. doi: 10.1016/0165-2427(90)90064-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989 Aug;63(8):3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes passively protect against gastroenteritis in suckling mice. J Virol. 1990 Dec;64(12):6325–6328. doi: 10.1128/jvi.64.12.6325-6328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham G., Bridger J. C. The effect of dexamethasone-induced immunosuppression on the development of faecal antibody and recovery from and resistance to rotavirus infection. Vet Immunol Immunopathol. 1992 Apr;32(1-2):77–92. doi: 10.1016/0165-2427(92)90070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons K. R., Howard C. J., Jones B. V., Sopp P. Investigation of bovine gut associated lymphoid tissue (GALT) using monoclonal antibodies against bovine lymphocytes. Vet Pathol. 1989 Sep;26(5):396–408. doi: 10.1177/030098588902600505. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Lodmell D. L. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991 Jul;65(7):3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock D. H. Characterisation of rotavirus isolates from sub-clinically infected calves by genome profile analysis. Vet Microbiol. 1987 Jan;13(1):27–34. doi: 10.1016/0378-1135(87)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J. Development of nasal, fecal and serum isotype-specific antibodies in calves challenged with bovine coronavirus or rotavirus. Vet Immunol Immunopathol. 1987 Dec;17(1-4):425–439. doi: 10.1016/0165-2427(87)90159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Terzolo H. R., Sherwood D., Campbell I., Menzies J. D., Synge B. A. Aetiology of diarrhoea in young calves. Vet Rec. 1986 Jul 12;119(2):31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- Totterdell B. M., Banatvala J. E., Chrystie I. L., Ball G., Cubitt W. D. Systemic lymphoproliferative responses to rotavirus. J Med Virol. 1988 May;25(1):37–44. doi: 10.1002/jmv.1890250106. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Ijzerman J., De Leeuw P. W. Intestinal antibody response after vaccination and infection with rotavirus of calves fed colostrum with or without rotavirus antibody. Vet Immunol Immunopathol. 1986 Jan;11(1):45–63. doi: 10.1016/0165-2427(86)90087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu Rev Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Brubaker J. O., Vargas-Cortes M., O'Donnell C. L. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991 May 1;173(5):1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]


