Abstract
Cell cultures expressing a retroviral envelope are relatively resistant to superinfection by retroviruses which bear envelopes using the same receptor. We tested whether this phenomenon, known as interference to superinfection, might confer protection against retroviral diseases. Newborn mice first inoculated with the attenuated strain B3 of Friend murine leukemia virus (F-MuLV) were protected against severe early hemolytic anemia and nonacute anemiant erythroleukemia induced by the virulent strain 57 of F-MuLV. Vaccinated animals were also protected as adults against acute polycythemic erythroleukemia induced upon inoculation with the viral complex containing the defective spleen focus-forming virus and F-MuLV 57 as helper virus. Animals were inoculated as newborns, which is known to induce immune tolerance in mice, and the rapid kinetics of protection, incompatible with the delay necessary for the immune response to develop, indicated that protection was not due to an immune mechanism but rather was due to the rapid and long-lasting phenomenon of interference. This result was confirmed by combining parental and envelope chimeric MuLV from different interference groups as vaccinal and challenge viruses. Although efficient protection could be provided by vaccination by interference, we observed that attenuated replication-competent retroviruses from heterologous interference groups might exert deleterious synergistic effects.
Full text
PDF
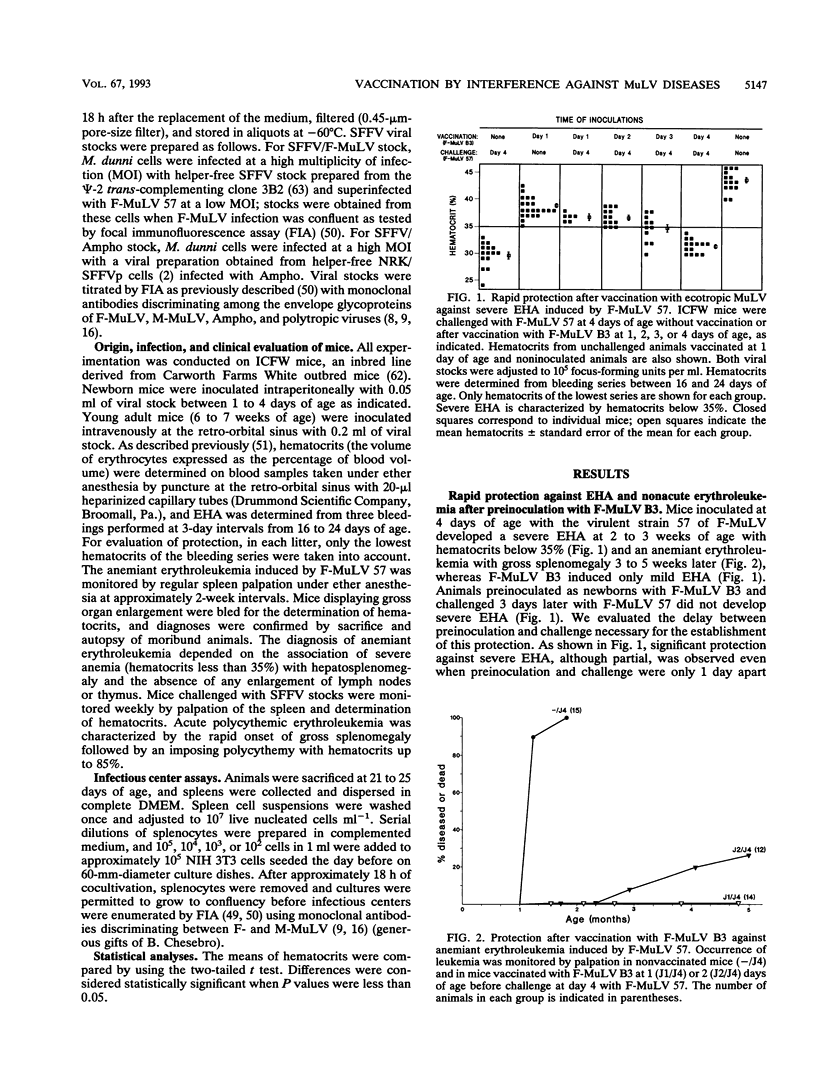
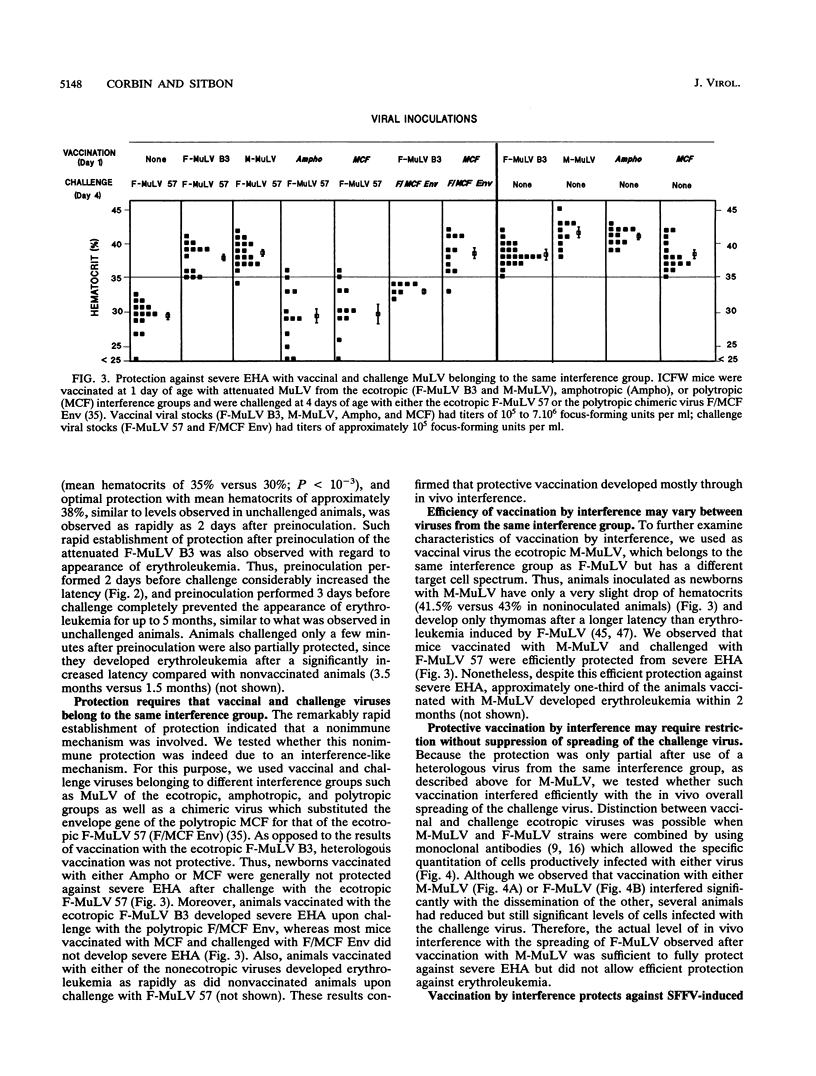
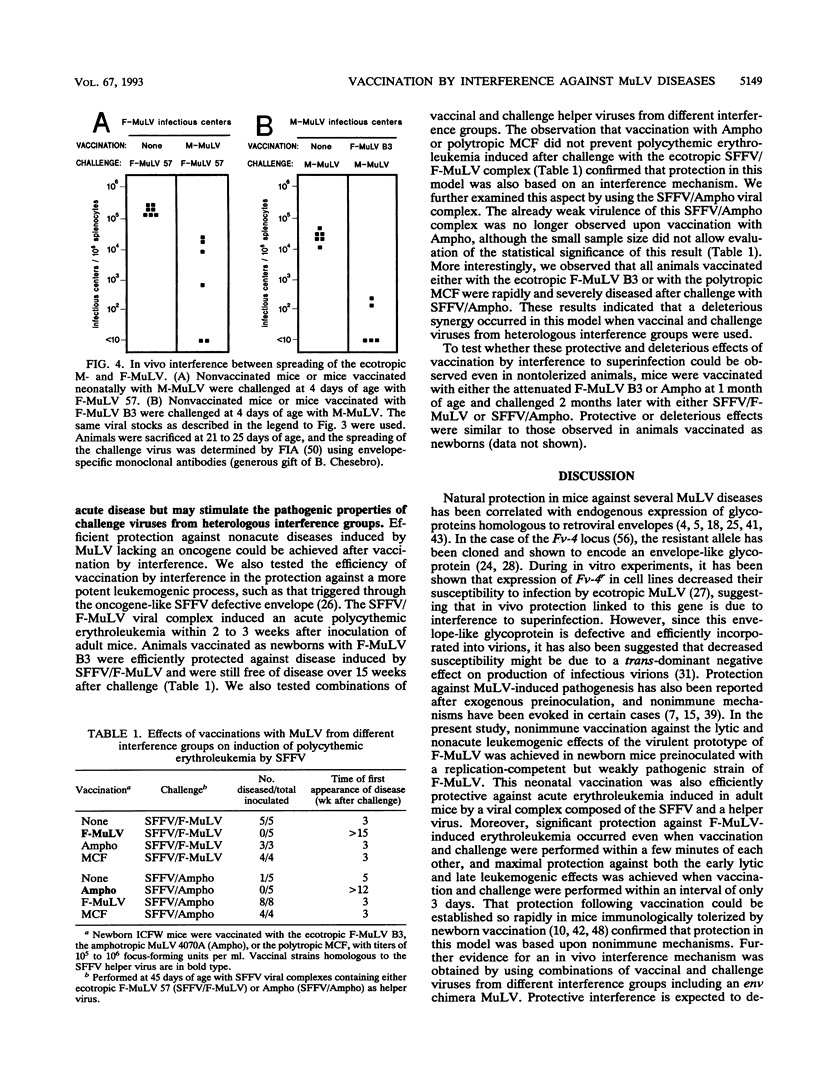



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Troxler D. H., Scolnick E. M., Aaronson S. A. Analysis of translational products of Friend strain of spleen focus-forming virus. J Virol. 1978 Sep;27(3):826–830. doi: 10.1128/jvi.27.3.826-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. S., Sitbon M., Portis J. L. The endogenous mink cell focus-forming (MCF) gp70 linked to the Rmcf gene restricts MCF virus replication in vivo and provides partial resistance to erythroleukemia induced by Friend murine leukemia virus. J Exp Med. 1988 May 1;167(5):1535–1546. doi: 10.1084/jem.167.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. S., Wehrly K., Portis J. L., Chesebro B. Host genes conferring resistance to a central nervous system disease induced by a polytropic recombinant Friend murine retrovirus. J Virol. 1990 Feb;64(2):493–498. doi: 10.1128/jvi.64.2.493-498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösze Z., Thiesen H. J., Charnay P. A transcriptional enhancer with specificity for erythroid cells is located in the long terminal repeat of the Friend murine leukemia virus. EMBO J. 1986 Jul;5(7):1615–1623. doi: 10.1002/j.1460-2075.1986.tb04404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Morse H. C., 3rd, Makino M., Ruscetti S. K., Hartley J. W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1989 May;86(10):3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Portis J. L., Wehrly K., Nishio J. Effect of murine host genotype on MCF virus expression, latency, and leukemia cell type of leukemias induced by Friend murine leukemia helper virus. Virology. 1983 Jul 15;128(1):221–233. doi: 10.1016/0042-6822(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Collavo D., Zanovello P., Biasi G., Chieco-Bianchi L. T lymphocyte tolerance and early appearance of virus-induced cell surface antigens in Moloney-murine leukemia virus neonatally injected mice. J Immunol. 1981 Jan;126(1):187–193. [PubMed] [Google Scholar]
- Crittenden L. B., McMahon S., Halpern M. S., Fadly A. M. Embryonic infection with the endogenous avian leukosis virus Rous-associated virus-0 alters responses to exogenous avian leukosis virus infection. J Virol. 1987 Mar;61(3):722–725. doi: 10.1128/jvi.61.3.722-725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- De Rossi A., D'Andrea E., Biasi G., Collavo D., Chieco-Bianchi L. Protection from spontaneous lymphoma development in SJL/J(v+) mice neonatally injected with dualtropic SJL-151 virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2775–2779. doi: 10.1073/pnas.80.9.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Morrison R. P., Malik F. G., Portis J., Britt W. J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990 Dec;64(12):6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. 3. DETERMINING INFLUENCE OF A NEW HELPER VIRUS ON THE HOST RANGE AND SUSCEPTIBILITY TO INTERFERENCE OF RSV. Virology. 1965 Feb;25:248–255. doi: 10.1016/0042-6822(65)90203-5. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991 Aug;65(8):4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. Cellular expression of murine leukemia virus gp70-related antigen on thymocytes of uninfected mice correlates with Fv-4 gene-controlled resistance to Friend leukemia virus infection. Virology. 1983 Jul 15;128(1):127–139. doi: 10.1016/0042-6822(83)90324-0. [DOI] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Kai K., Sato H., Odaka T. Relationship between the cellular resistance to Friend murine leukemia virus infection and the expression of murine leukemia virus-gp70-related glycoprotein on cell surface of BALB/c-Fv-4wr mice. Virology. 1986 Apr 30;150(2):509–512. doi: 10.1016/0042-6822(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Gromet N. J., Ikeda H., Buckler C. E. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc Natl Acad Sci U S A. 1984 Feb;81(3):834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Menke J. G., Scolnick E. M. Recovery of biologically active spleen focus-forming virus from molecularly cloned spleen focus-forming virus-pBR322 circular DNA by cotransfection with infectious type C retroviral DNA. J Virol. 1980 Sep;35(3):710–721. doi: 10.1128/jvi.35.3.710-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Yoshikura H. Construction and characterization of the recombinant Moloney murine leukemia viruses bearing the mouse Fv-4 env gene. J Virol. 1990 Mar;64(3):1033–1043. doi: 10.1128/jvi.64.3.1033-1043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T., Risser R. Interference established in mice by infection with Friend murine leukemia virus. J Virol. 1992 Sep;66(9):5696–5702. doi: 10.1128/jvi.66.9.5696-5702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., McKinney M. D., Agranovsky O. Contribution of the gag and pol sequences to the leukemogenicity of Friend murine leukemia virus. J Virol. 1985 Jun;54(3):864–868. doi: 10.1128/jvi.54.3.864-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P. Resistance of mice infected with Moloney leukemia virus to Friend virus infection. Science. 1963 Jul 5;141(3575):40–41. doi: 10.1126/science.141.3575.40. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Pal B. K., Gardner M. B. Characterization of a highly oncogenic murine leukemia virus from wild mice. Int J Cancer. 1982 Mar 15;29(3):345–350. doi: 10.1002/ijc.2910290319. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Feild J., Davis L., Oliff A. Factors determining the susceptibility of NIH swiss mice to erythroleukemia induced by Friend murine leukemia virus. Virology. 1982 Mar;117(2):357–365. doi: 10.1016/0042-6822(82)90475-5. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Matthai R., Potter M. Susceptibility of BALB/c mice carrying various DBA/2 genes to development of Friend murine leukemia virus-induced erythroleukemia. J Exp Med. 1985 Nov 1;162(5):1579–1587. doi: 10.1084/jem.162.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Cheong M. P., Hartley J. W., Huebner R. J. A viral interference test for mouse leukemia viruses. Virology. 1967 Sep;33(1):180–184. doi: 10.1016/0042-6822(67)90111-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Ellerbrok H., Pozo F., Nishio J., Hayes S. F., Evans L. H., Chesebro B. Sequences in the U5-gag-pol region influence early and late pathogenic effects of Friend and Moloney murine leukemia viruses. J Virol. 1990 May;64(5):2135–2140. doi: 10.1128/jvi.64.5.2135-2140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Evans L., Nishio J., Wehrly K., Chesebro B. Analysis of two strains of Friend murine leukemia viruses differing in ability to induce early splenomegaly: lack of relationship with generation of recombinant mink cell focus-forming viruses. J Virol. 1986 Jan;57(1):389–393. doi: 10.1128/jvi.57.1.389-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Chesebro B. Pseudotyping of dual-tropic recombinant viruses generated by infection of mice with different ecotropic murine leukemia viruses. Virology. 1985 Jan 15;140(1):144–151. doi: 10.1016/0042-6822(85)90453-2. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Lodmell D., Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985 Feb;141(1):110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Sitbon M., d'Auriol L., Ellerbrok H., André C., Nishio J., Perryman S., Pozo F., Hayes S. F., Wehrly K., Tambourin P. Substitution of leucine for isoleucine in a sequence highly conserved among retroviral envelope surface glycoproteins attenuates the lytic effect of the Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5932–5936. doi: 10.1073/pnas.88.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Golemis E., Fredrickson T. N., Hartley J. W., Hopkins N. Mutation of the core or adjacent LVb elements of the Moloney murine leukemia virus enhancer alters disease specificity. Genes Dev. 1990 Feb;4(2):233–242. doi: 10.1101/gad.4.2.233. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiesen H. J., Bösze Z., Henry L., Charnay P. A DNA element responsible for the different tissue specificities of Friend and Moloney retroviral enhancers. J Virol. 1988 Feb;62(2):614–618. doi: 10.1128/jvi.62.2.614-618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Yuan E., Linemeyer D., Ruscetti S., Scolnick E. M. Helper-independent mink cell focus-inducing strains of Friend murine type-C virus: potential relationship to the origin of replication-defective spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):639–653. doi: 10.1084/jem.148.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965 Aug;26(4):664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Naito Y., Moriwaki K. Unstable resistance of G mouse fibroblasts to ecotropic murine leukemia virus infection. J Virol. 1979 Mar;29(3):1078–1086. doi: 10.1128/jvi.29.3.1078-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


