Abstract
Infections caused by lentiviruses, including human immunodeficiency virus, are characterized by slowly progressive disease in the presence of a virus-specific immune response. The earliest events in the virus-host interaction are likely to be important in determining disease establishment and progression, and the kinetics of these early events following lentiviral infection are described here. Lymphatic cannulation in the sheep has been used to monitor both the virus and the immune response in efferent lymph after infection of the node with maedi-visna virus (MVV). Viral replication and dissemination could be detected and consisted of a wave of MVV-infected cells leaving the node around 9 to 18 days postinfection. No cell-free virus was recovered despite the fact that soluble MVV p25 was detected in lymph plasma. The maximum frequency of MVV-infected cells was only 11 in 10(6) but over the first 20 days of infection amounted to greater than 10(4) virus-infected cells leaving the node. There was a profound increase in the output of activated lymphoblast from the lymph nodes of infected sheep, characterized by an increased percentage of CD8+ lymphoblasts. All of the CD8+ lymphoblasts at the peak of the response expressed both major histocompatibility complex class II DR and DQ molecules but not interleukin-2 receptor (CD25). The in vitro proliferative response of efferent lymph cells existing the node after challenge with MVV to both recombinant human interleukin-2 and the mitogen concanavalin A was decreased between days 8 and 16 postinfection, and a specific proliferative response to MVV was not detected until after day 15. Despite the high level of CD8+ lymphoblasts in efferent lymph, direct MVV-specific cytotoxic activity was demonstrated in only one of the five MVV-challenged sheep. MVV-specific antibody responses, including neutralization and MVV p25 immune complexes in efferent lymph, were detectable during the major period of virus dissemination. The relationship of these findings to the evasion of the host's acute immune response by MVV is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autran B., Sadat-Sowti B., Hadida F., Parrot A., Guillon J. M., Plata F., Mayaud C., Debré P. HIV-specific cytotoxic T lymphocytes against alveolar macrophages: specificities and downregulation. Res Virol. 1991 Mar-Jun;142(2-3):113–118. doi: 10.1016/0923-2516(91)90046-6. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Bujdoso R., Young P., Harkiss G. D., McConnell I. Antigen presentation in the sheep: generation of antigen-specific T-cell lines. Immunology. 1989 Apr;66(4):559–564. [PMC free article] [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Deng P., Cutlip R. C., Lehmkuhl H. D., Brogden K. A. Ultrastructure and frequency of mastitis caused by ovine progressive pneumonia virus infection in sheep. Vet Pathol. 1986 Mar;23(2):184–189. doi: 10.1177/030098588602300212. [DOI] [PubMed] [Google Scholar]
- Dutia B. M., Hopkins J., Allington M. P., Bujdoso R., McConnell I. Characterization of monoclonal antibodies specific for alpha- and beta-chains of sheep MHC class II. Immunology. 1990 May;70(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N. Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol. 1991 Sep;65(9):4902–4909. doi: 10.1128/jvi.65.9.4902-4909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D. H., Hess J. L., Small J. A., Clements J. E. Regulation of the visna virus long terminal repeat in macrophages involves cellular factors that bind sequences containing AP-1 sites. Mol Cell Biol. 1989 Jun;9(6):2728–2733. doi: 10.1128/mcb.9.6.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Ventura P., Stowring L., Haase A. T. Quantitative analysis of visna virus replication in vivo. Virology. 1985 Feb;141(1):148–154. doi: 10.1016/0042-6822(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kara C. J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- Gorrell M. D., Brandon M. R., Sheffer D., Adams R. J., Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992 May;66(5):2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F. M., Nixon D. F., Alp N., McMichael A. J., Borysiewicz L. K. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2(8):707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M., Nelson D. L., Waldmann T. A. The human interleukin-2 receptor: normal and abnormal expression in T cells and in leukemias induced by the human T-lymphotropic retroviruses. Ann Intern Med. 1986 Oct;105(4):560–572. doi: 10.7326/0003-4819-105-4-560. [DOI] [PubMed] [Google Scholar]
- Gudnadóttir M. Visna-maedi in sheep. Prog Med Virol. 1974;18(0):336–349. [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hall J. G. A method for collecting lymph from the prefemoral lymph node of unanaesthetised sheep. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):200–205. doi: 10.1113/expphysiol.1967.sp001902. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hom R. C., Finberg R. W., Mullaney S., Ruprecht R. M. Protective cellular retroviral immunity requires both CD4+ and CD8+ immune T cells. J Virol. 1991 Jan;65(1):220–224. doi: 10.1128/jvi.65.1.220-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B. Characteristics of lymphoblasts appearing in efferent lymph in response to immunization with vaccinia virus. Immunology. 1985 Sep;56(1):23–31. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B. Kinetics of cytotoxic lymphocytes in efferent lymph from single lymph nodes following immunization with vaccinia virus. Clin Exp Immunol. 1984 Jun;56(3):515–523. [PMC free article] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Karasek M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J Invest Dermatol. 1978 Aug;71(2):157–162. doi: 10.1111/1523-1747.ep12546943. [DOI] [PubMed] [Google Scholar]
- Lowe J., Bird P., Hardie D., Jefferis R., Ling N. R. Monoclonal antibodies (McAbs) to determinants on human gamma chains: properties of antibodies showing subclass restriction or subclass specificity. Immunology. 1982 Oct;47(2):329–336. [PMC free article] [PubMed] [Google Scholar]
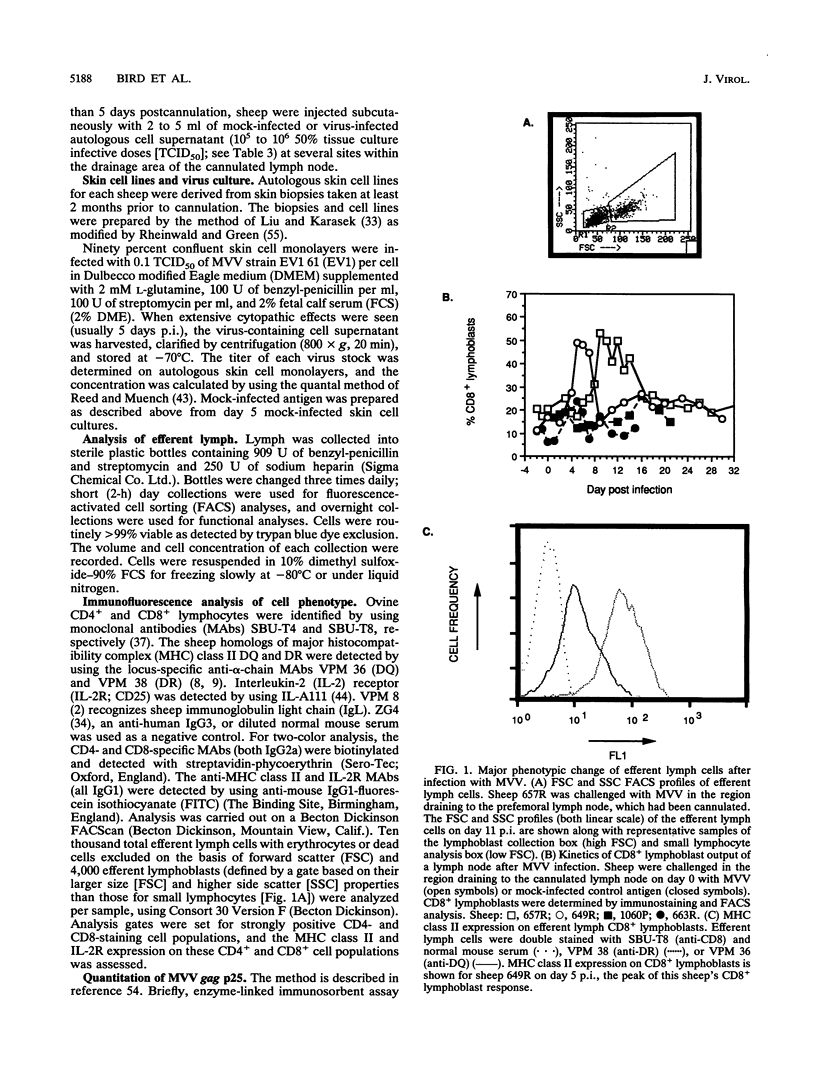
- Lynch F., Doherty P. C., Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989 May 15;142(10):3592–3598. [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- McConnell I., Hopkins J. Lymphocyte traffic through antigen-stimulated lymph nodes. I. Complement activation within lymph nodes initiates cell shutdown. Immunology. 1981 Feb;42(2):217–223. [PMC free article] [PubMed] [Google Scholar]
- McCune J. M. HIV-1: the infective process in vivo. Cell. 1991 Jan 25;64(2):351–363. doi: 10.1016/0092-8674(91)90644-e. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Adams D. S., Johnson G. C., Klevjer-Anderson P., Barbee D. D., Gorham J. R. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am J Vet Res. 1986 Mar;47(3):537–540. [PubMed] [Google Scholar]
- Naessens J., Sileghem M., MacHugh N., Park Y. H., Davis W. C., Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology. 1992 Jun;76(2):305–309. [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Martin J. R., Georgsson G., Palsson P. A., Lutley R. E., Petursson G. The effect of post-infection immunization on the severity of experimental visna. J Comp Pathol. 1981 Apr;91(2):185–191. doi: 10.1016/0021-9975(81)90023-2. [DOI] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Koenig S., Baseler M., Lane H. C., Fauci A. S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25- T cell population. J Immunol. 1990 Mar 1;144(5):1696–1704. [PubMed] [Google Scholar]
- Reimann K. A., Snyder G. B., Chalifoux L. V., Waite B. C., Miller M. D., Yamamoto H., Spertini O., Letvin N. L. An activated CD8+ lymphocyte appears in lymph nodes of rhesus monkeys early after infection with simian immunodeficiency virus. J Clin Invest. 1991 Oct;88(4):1113–1120. doi: 10.1172/JCI115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
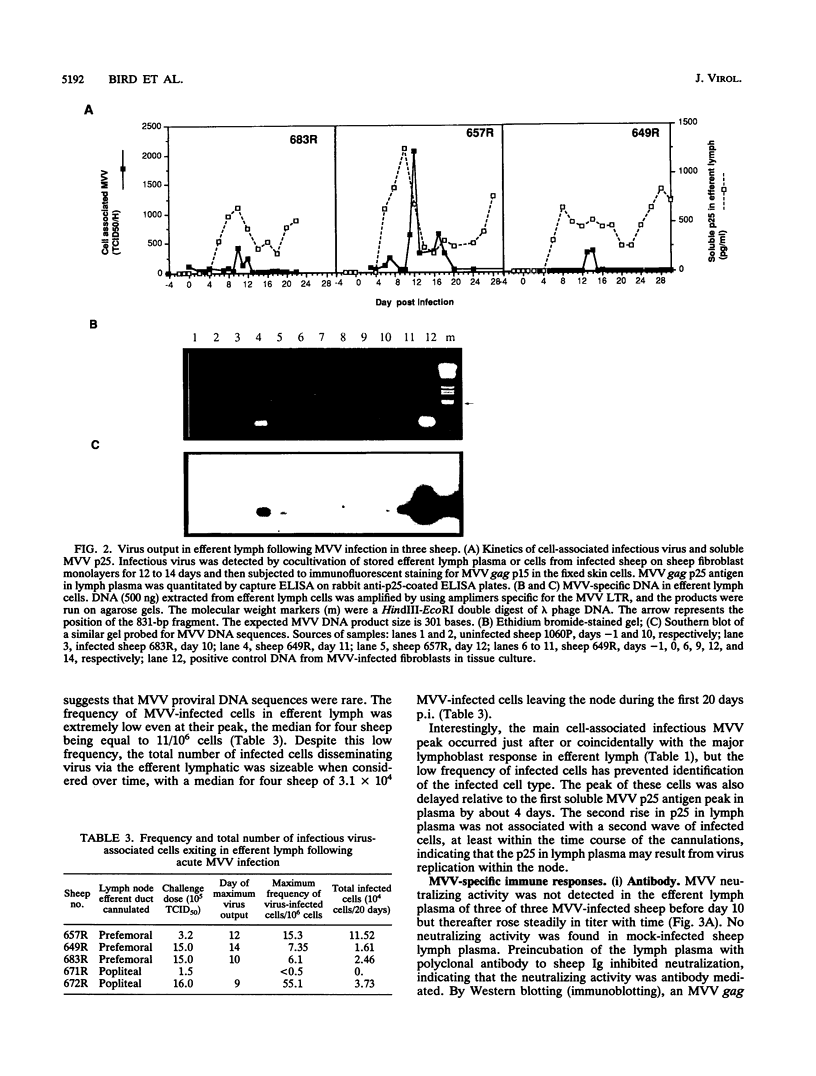
- Reyburn H. T., Roy D. J., Blacklaws B. A., Sargan D. R., McConnell I. Expression of maedi-visna virus major core protein, p25: development of a sensitive p25 antigen detection assay. J Virol Methods. 1992 Jun;37(3):305–320. doi: 10.1016/0166-0934(92)90031-8. [DOI] [PubMed] [Google Scholar]
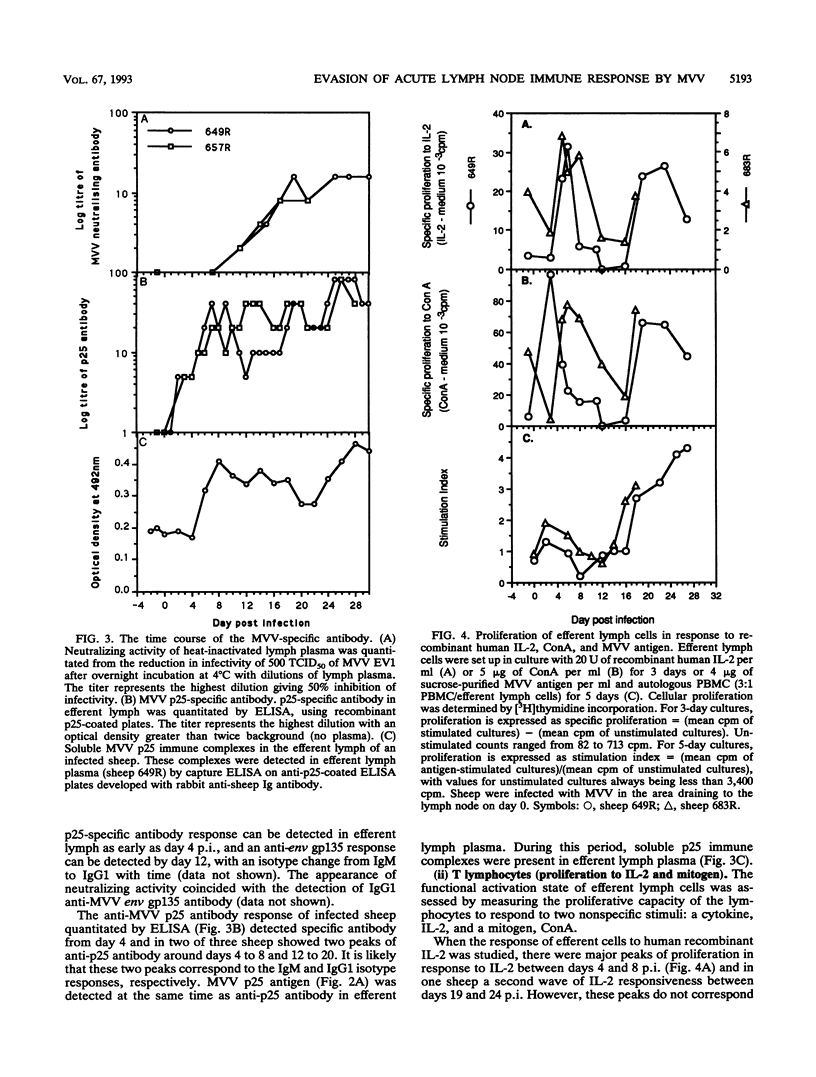
- Reyburn H. T., Roy D. J., Blacklaws B. A., Sargan D. R., Watt N. J., McConnell I. Characteristics of the T cell-mediated immune response to maedi-visna virus. Virology. 1992 Dec;191(2):1009–1012. doi: 10.1016/0042-6822(92)90282-t. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
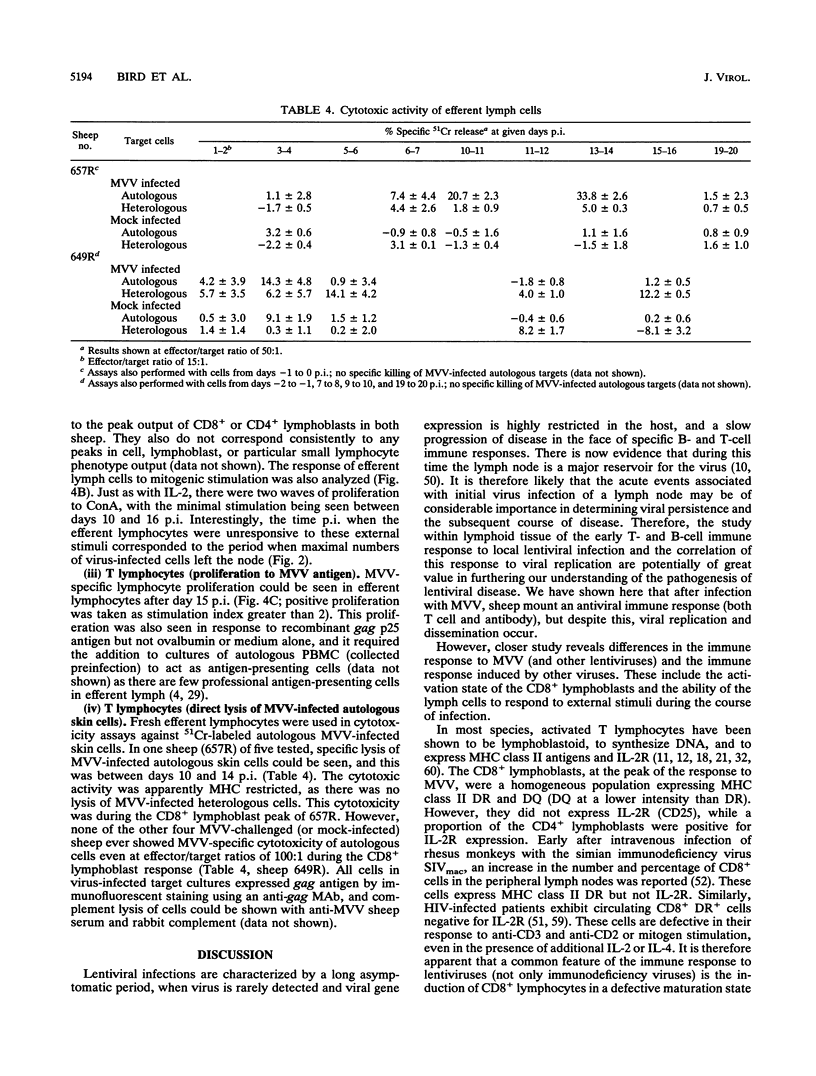
- Sadat-Sowti B., Debré P., Idziorek T., Guillon J. M., Hadida F., Okzenhendler E., Katlama C., Mayaud C., Autran B. A lectin-binding soluble factor released by CD8+CD57+ lymphocytes from AIDS patients inhibits T cell cytotoxicity. Eur J Immunol. 1991 Mar;21(3):737–741. doi: 10.1002/eji.1830210329. [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez J. F., Moody D. J., Giorgi J. V., Martinez-Maza O., Mitsuyasu R. T., Fahey J. L. Reduced ecto-5'-nucleotidase activity and enhanced OKT10 and HLA-DR expression on CD8 (T suppressor/cytotoxic) lymphocytes in the acquired immune deficiency syndrome: evidence of CD8 cell immaturity. J Immunol. 1985 Sep;135(3):1778–1785. [PubMed] [Google Scholar]
- Salvadori S., Pizzimenti A., Cohen S., Zier K. S. The control of class II expression on T cells is independent of the regulation of Tac and the induction of proliferation. Clin Exp Immunol. 1991 Dec;86(3):544–549. doi: 10.1111/j.1365-2249.1991.tb02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargan D. R., Bennet I. D., Cousens C., Roy D. J., Blacklaws B. A., Dalziel R. G., Watt N. J., McConnell I. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J Gen Virol. 1991 Aug;72(Pt 8):1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Morris B. The response of the popliteal lymph node of the sheep to swine influenza virus. Aust J Exp Biol Med Sci. 1970 Feb;48(1):33–46. doi: 10.1038/icb.1970.4. [DOI] [PubMed] [Google Scholar]
- Trnka Z., Cahill R. N. Aspects of the immune response in single lymph nodes. Monogr Allergy. 1980;16:245–259. [PubMed] [Google Scholar]
- Wendler I., Bienzle U., Hunsmann G. Neutralizing antibodies and the course of HIV-induced disease. AIDS Res Hum Retroviruses. 1987 Summer;3(2):157–163. doi: 10.1089/aid.1987.3.157. [DOI] [PubMed] [Google Scholar]