Abstract
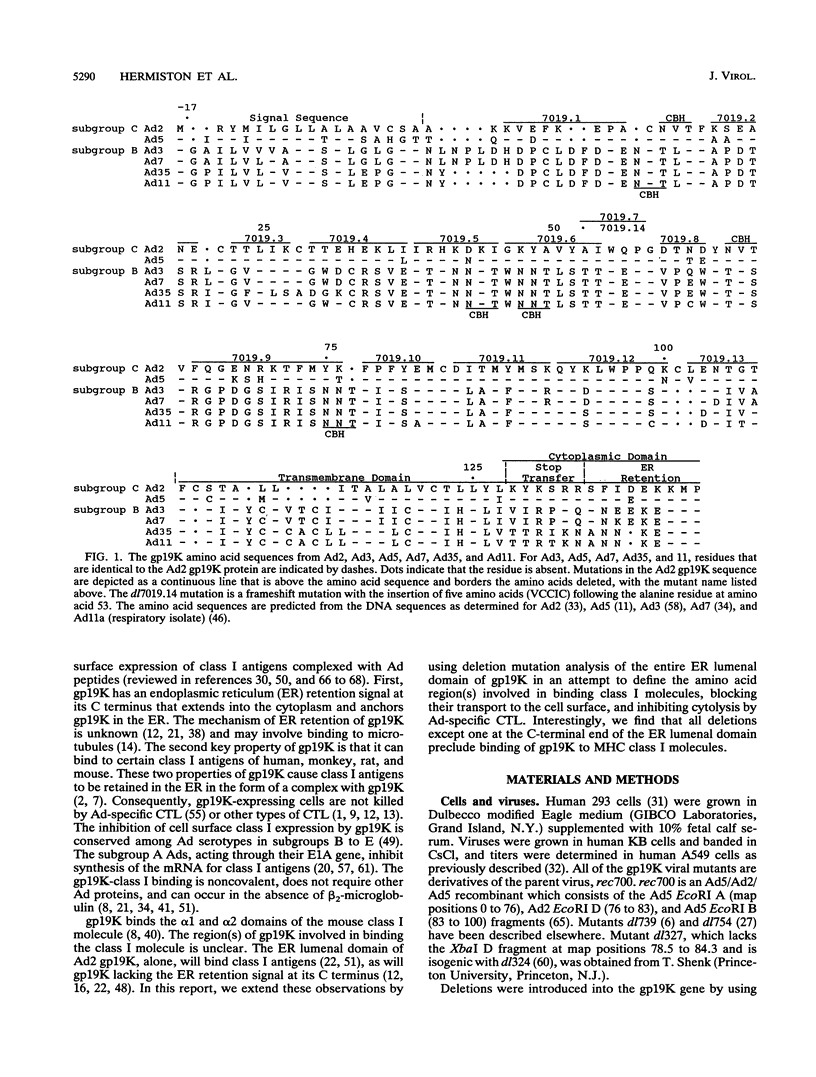
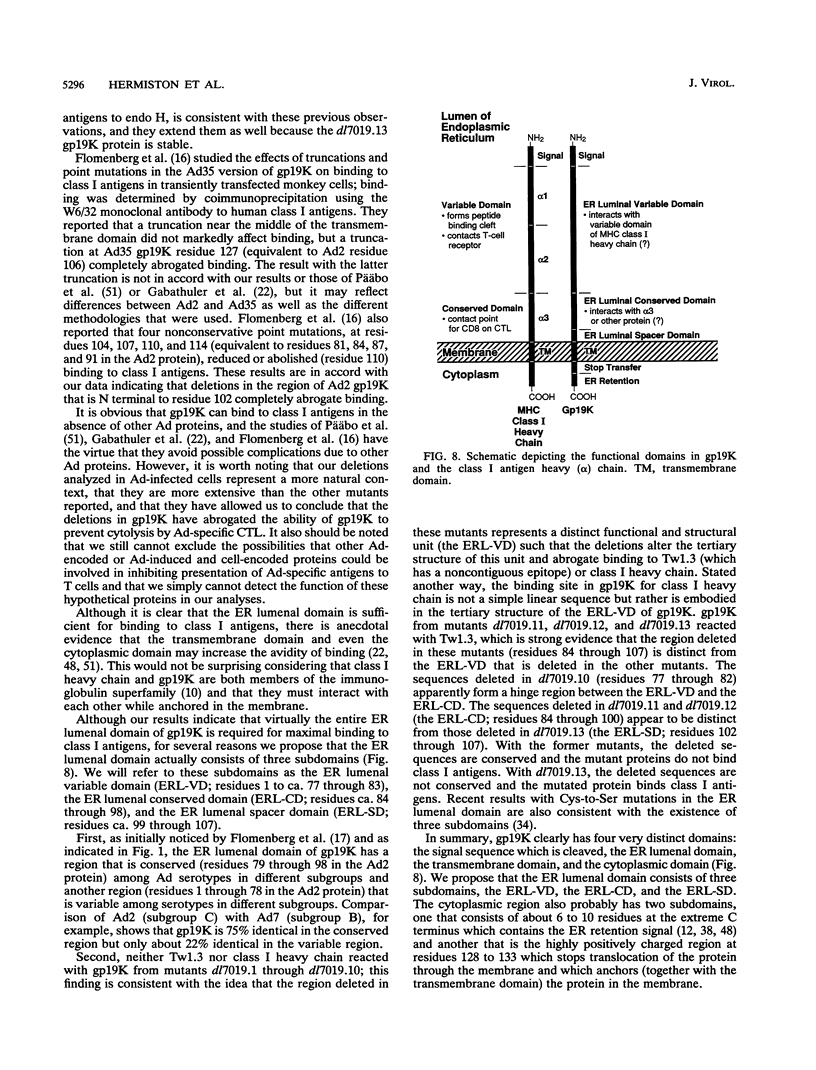
Adenovirus E3-gp19K is a transmembrane glycoprotein, localized in the endoplasmic reticulum (ER), which forms a complex with major histocompatibility complex (MHC) class I antigens and retains them in the ER, thereby preventing cytolysis by cytotoxic T lymphocytes (CTL). The ER lumenal domain of gp19K, residues 1 to 107, is known to be sufficient for binding to class I antigens; the transmembrane and cytoplasmic ER retention domains are located at residues ca. 108 to 127 and 128 to 142, respectively. To identify more precisely which gp19K regions are involved in binding to class I antigens, we constructed 13 in-frame virus deletion mutants (4 to 12 amino acids deleted) in the ER lumenal domain of gp19K, and we analyzed the ability of the mutant proteins to form a complex with class I antigens, retain them in the ER, and prevent cytolysis by adenovirus-specific CTL. All mutant proteins except one (residues 102 to 107 deleted) were defective for these properties, indicating that the ability of gp19K to bind to class I antigens is highly sensitive to mutation. All mutant proteins were stable and were retained in the ER. Sequence comparisons among adenovirus serotypes reveal that the ER lumenal domain of gp19K consists of a variable region (residues 1 to 76) and a conserved region (residues 77 to 98). We show, using the mutant proteins, that the gp19K-specific monoclonal antibody Tw1.3 recognizes a noncontiguous epitope in the variable region and that disruption of the variable region by deletion destroys the epitope. The monoclonal antibody and class I antigen binding results, together with the serotype sequence comparisons, are consistent with the idea that the ER lumenal domain of gp19K has three subdomains that we have termed the ER lumenal variable domain (residues 1 to ca. 77 to 83), the ER lumenal conserved domain (residues ca. 84 to 98), and the ER lumenal spacer domain (residues 99 to 107). We suggest that the ER lumenal variable domain of gp19K has a specific tertiary structure that is important for binding to the polymorphic alpha 1 and alpha 2 domains of class I heavy (alpha) chains. We suggest that the ER lumenal conserved domain of gp19K may interact with some conserved protein, perhaps the highly conserved alpha 3 domain of class I heavy chains. Finally, the ER lumenal spacer domain may allow the ER lumenal variable and conserved domains to extend out from the ER membrane so that they can interact with class I heavy chains.
Full text
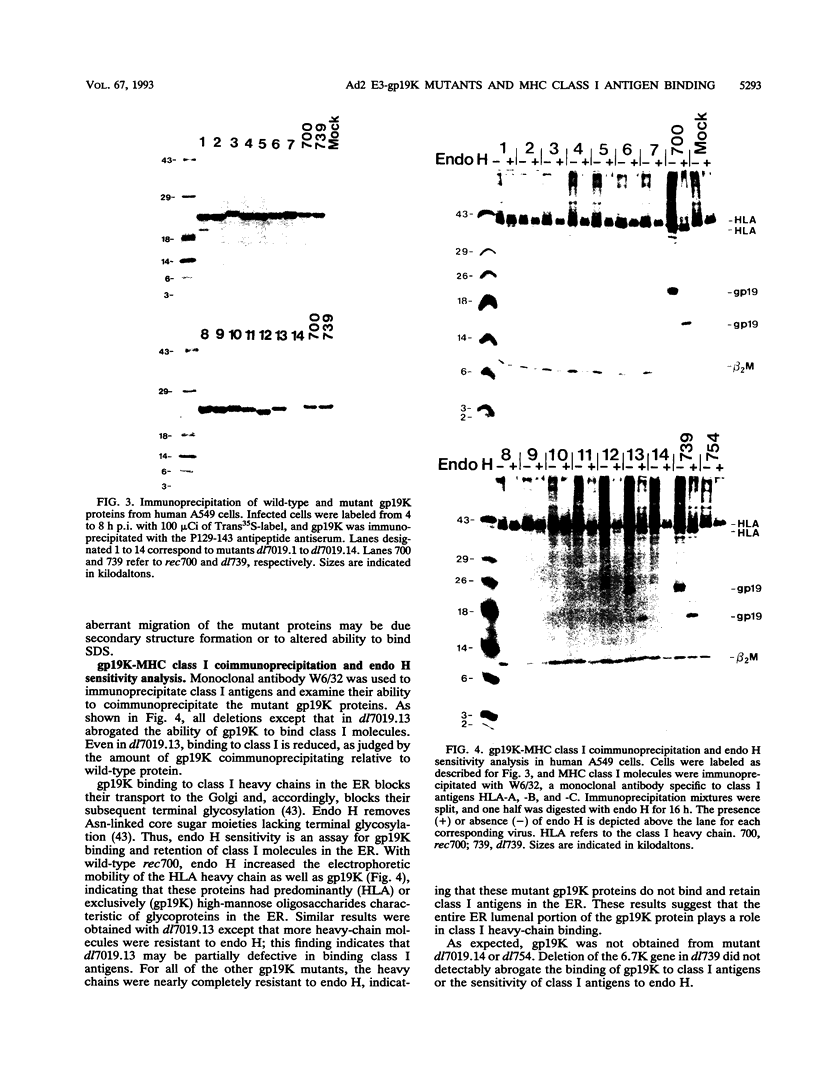
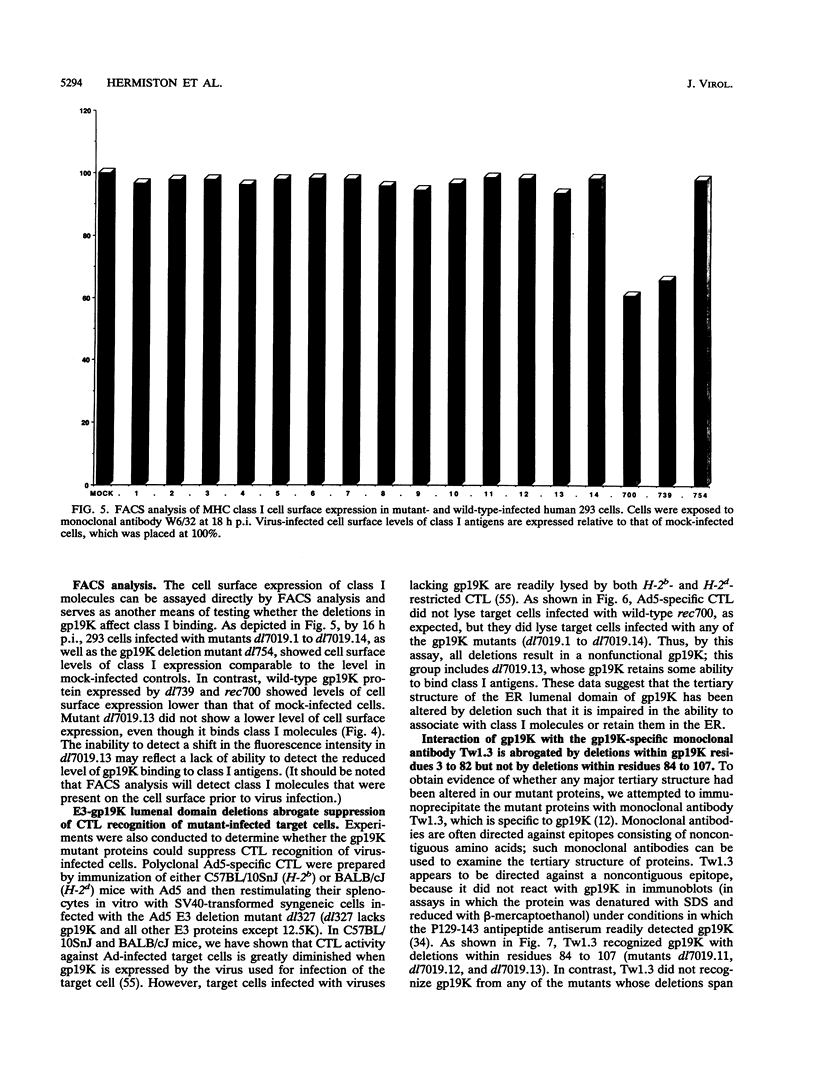
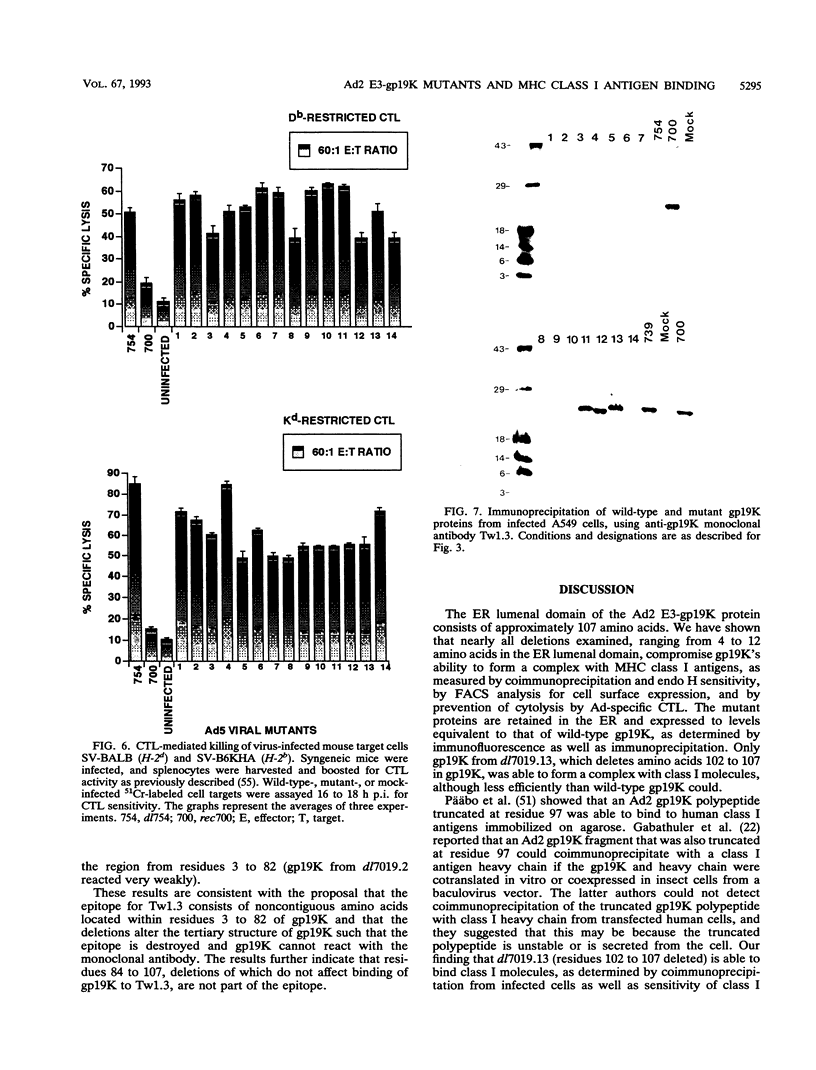
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., McMichael A., Peterson P. A. Reduced allorecognition of adenovirus-2 infected cells. J Immunol. 1987 Jun 1;138(11):3960–3966. [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brady H. A., Scaria A., Wold W. S. Map of cis-acting sequences that determine alternative pre-mRNA processing in the E3 complex transcription unit of adenovirus. J Virol. 1992 Oct;66(10):5914–5923. doi: 10.1128/jvi.66.10.5914-5923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Maizel J. V., Jr Homology of adenoviral E3 glycoprotein with HLA-DR heavy chain. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6039–6043. doi: 10.1073/pnas.81.19.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Bennink J. R., Yewdell J. W. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991 Dec 1;174(6):1629–1637. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Dahllöf B., Wallin M., Kvist S. The endoplasmic reticulum retention signal of the E3/19K protein of adenovirus-2 is microtubule binding. J Biol Chem. 1991 Jan 25;266(3):1804–1808. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Flomenberg P. R., Chen M., Horwitz M. S. Sequence and genetic organization of adenovirus type 35 early region 3. J Virol. 1988 Nov;62(11):4431–4437. doi: 10.1128/jvi.62.11.4431-4437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flomenberg P., Szmulewicz J., Gutierrez E., Lupatkin H. Role of the adenovirus E3-19k conserved region in binding major histocompatibility complex class I molecules. J Virol. 1992 Aug;66(8):4778–4783. doi: 10.1128/jvi.66.8.4778-4783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. P., Hall C. E., Cooney M. K. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977 Apr;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Friedman D. J., Ricciardi R. P. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology. 1988 Jul;165(1):303–305. doi: 10.1016/0042-6822(88)90689-7. [DOI] [PubMed] [Google Scholar]
- Gabathuler R., Kvist S. The endoplasmic reticulum retention signal of the E3/19K protein of adenovirus type 2 consists of three separate amino acid segments at the carboxy terminus. J Cell Biol. 1990 Nov;111(5 Pt 1):1803–1810. doi: 10.1083/jcb.111.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabathuler R., Lévy F., Kvist S. Requirements for the association of adenovirus type 2 E3/19K wild-type and mutant proteins with HLA antigens. J Virol. 1990 Aug;64(8):3679–3685. doi: 10.1128/jvi.64.8.3679-3685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin K., Krishna S., Ponchel F., Frohlich M., Cummings D. E., Carlson R., Wands J. R., Isselbacher K. J., Pillai S., Ozturk M. The major histocompatibility complex class I antigen-binding protein p88 is the product of the calnexin gene. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8452–8456. doi: 10.1073/pnas.89.18.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Ranheim T. S., Tollefson A. E., Aquino L., Duerksen-Hughes P., Horton T. M., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol. 1991 Aug;65(8):4114–4123. doi: 10.1128/jvi.65.8.4114-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Sofola I. O., Tollefson A. E., Duerksen-Hughes P., Wold W. S. The adenovirus E3-14.7K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J Immunol. 1990 Nov 1;145(9):3080–3086. [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Wold W. S. Molecular mechanisms by which adenoviruses counteract antiviral immune defenses. Crit Rev Immunol. 1990;10(1):53–71. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Horton T. M., Ranheim T. S., Aquino L., Kusher D. I., Saha S. K., Ware C. F., Wold W. S., Gooding L. R. Adenovirus E3 14.7K protein functions in the absence of other adenovirus proteins to protect transfected cells from tumor necrosis factor cytolysis. J Virol. 1991 May;65(5):2629–2639. doi: 10.1128/jvi.65.5.2629-2639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T. M., Tollefson A. E., Wold W. S., Gooding L. R. A protein serologically and functionally related to the group C E3 14,700-kilodalton protein is found in multiple adenovirus serotypes. J Virol. 1990 Mar;64(3):1250–1255. doi: 10.1128/jvi.64.3.1250-1255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Burgert H. G. E3/19K from adenovirus 2 is an immunosubversive protein that binds to a structural motif regulating the intracellular transport of major histocompatibility complex class I proteins. J Exp Med. 1990 Dec 1;172(6):1653–1664. doi: 10.1084/jem.172.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Wold W. S. Structures of the oligosaccharides of the glycoprotein coded by early region E3 of adenovirus 2. J Virol. 1981 Nov;40(2):440–449. doi: 10.1128/jvi.40.2.440-449.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpe O., Bellgrau D., Hammerling U., Lind P., Päbo S., Severinsson L., Peterson P. A. Complex formation of class I transplantation antigens and a viral glycoprotein. J Biol Chem. 1983 Sep 10;258(17):10594–10598. [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Mei Y. F., Wadell G. The nucleotide sequence of adenovirus type 11 early 3 region: comparison of genome type Ad11p and Ad11a. Virology. 1992 Nov;191(1):125–133. doi: 10.1016/0042-6822(92)90173-m. [DOI] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Parham P., Lomen C. E., Lawlor D. A., Ways J. P., Holmes N., Coppin H. L., Salter R. D., Wan A. M., Ennis P. D. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Severinsson L., Andersson M., Martens I., Nilsson T., Peterson P. A. Adenovirus proteins and MHC expression. Adv Cancer Res. 1989;52:151–163. doi: 10.1016/s0065-230x(08)60212-2. [DOI] [PubMed] [Google Scholar]
- Päbo S., Weber F., Nilsson T., Schaffner W., Peterson P. A. Structural and functional dissection of an MHC class I antigen-binding adenovirus glycoprotein. EMBO J. 1986 Aug;5(8):1921–1927. doi: 10.1002/j.1460-2075.1986.tb04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranheim T. S., Shisler J., Horton T. M., Wold L. J., Gooding L. R., Wold W. S. Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J Virol. 1993 Apr;67(4):2159–2167. doi: 10.1128/jvi.67.4.2159-2167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle F. C., Tollefson A. E., Wold W. S., Gooding L. R. Mouse anti-adenovirus cytotoxic T lymphocytes. Inhibition of lysis by E3 gp19K but not E3 14.7K. J Immunol. 1989 Sep 15;143(6):2031–2037. [PubMed] [Google Scholar]
- Severinsson L., Martens I., Peterson P. A. Differential association between two human MHC class I antigens and an adenoviral glycoprotein. J Immunol. 1986 Aug 1;137(3):1003–1009. [PubMed] [Google Scholar]
- Shemesh J., Rotem-Yehudar R., Ehrlich R. Transcriptional and posttranscriptional regulation of class I major histocompatibility complex genes following transformation with human adenoviruses. J Virol. 1991 Oct;65(10):5544–5548. doi: 10.1128/jvi.65.10.5544-5548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Region E3 of human adenoviruses; differences between the oncogenic adenovirus-3 and the non-oncogenic adenovirus-2. Gene. 1986;50(1-3):173–184. doi: 10.1016/0378-1119(86)90322-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia S. S. Differential effect of adenovirus 2 E3/19K glycoprotein on the expression of H-2Kb and H-2Db class I antigens and H-2Kb- and H-2Db-restricted SV40-specific CTL-mediated lysis. Virology. 1988 Aug;165(2):357–366. doi: 10.1016/0042-6822(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Wilson-Rawls J., Saha S. K., Krajcsi P., Tollefson A. E., Gooding L. R., Wold W. S. A 6700 MW membrane protein is encoded by region E3 of adenovirus type 2. Virology. 1990 Sep;178(1):204–212. doi: 10.1016/0042-6822(90)90395-8. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Deutscher S. L., Kapoor Q. S. The 19-kDa glycoprotein coded by region E3 of adenovirus. Purification, characterization, and structural analysis. J Biol Chem. 1985 Feb 25;260(4):2424–2431. [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Adenovirus region E3 proteins that prevent cytolysis by cytotoxic T cells and tumor necrosis factor. Mol Biol Med. 1989 Oct;6(5):433–452. [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]