Abstract
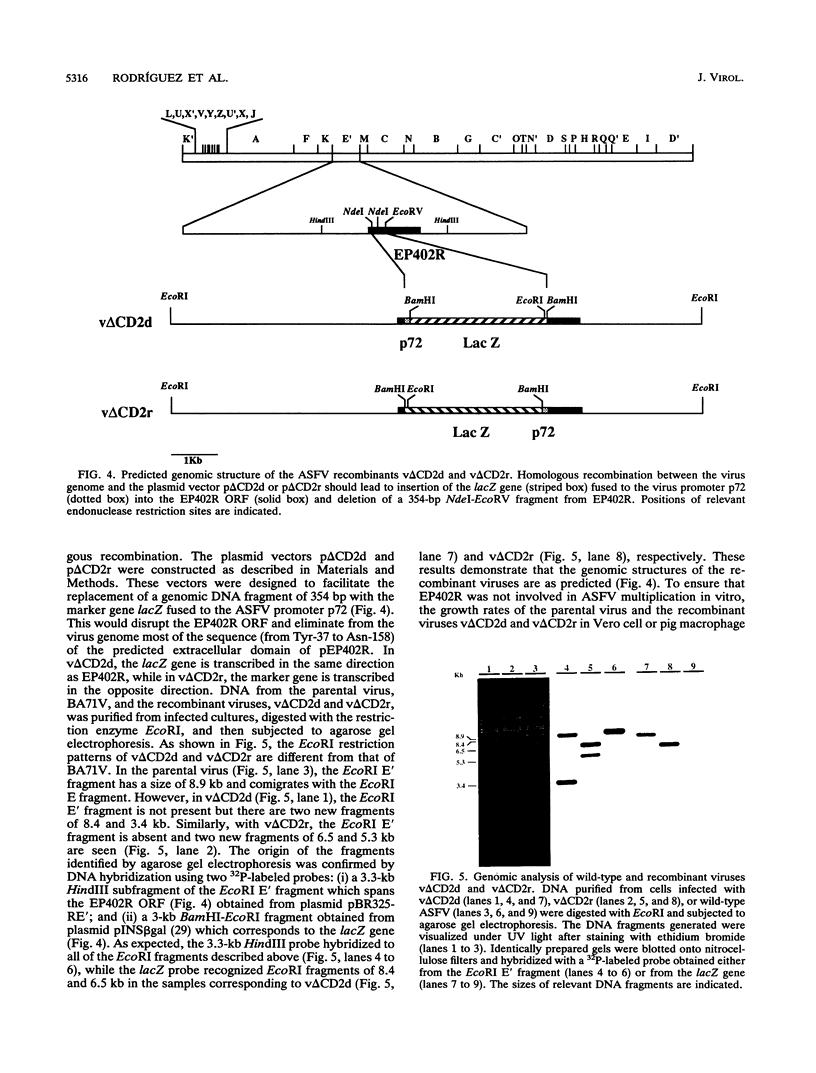
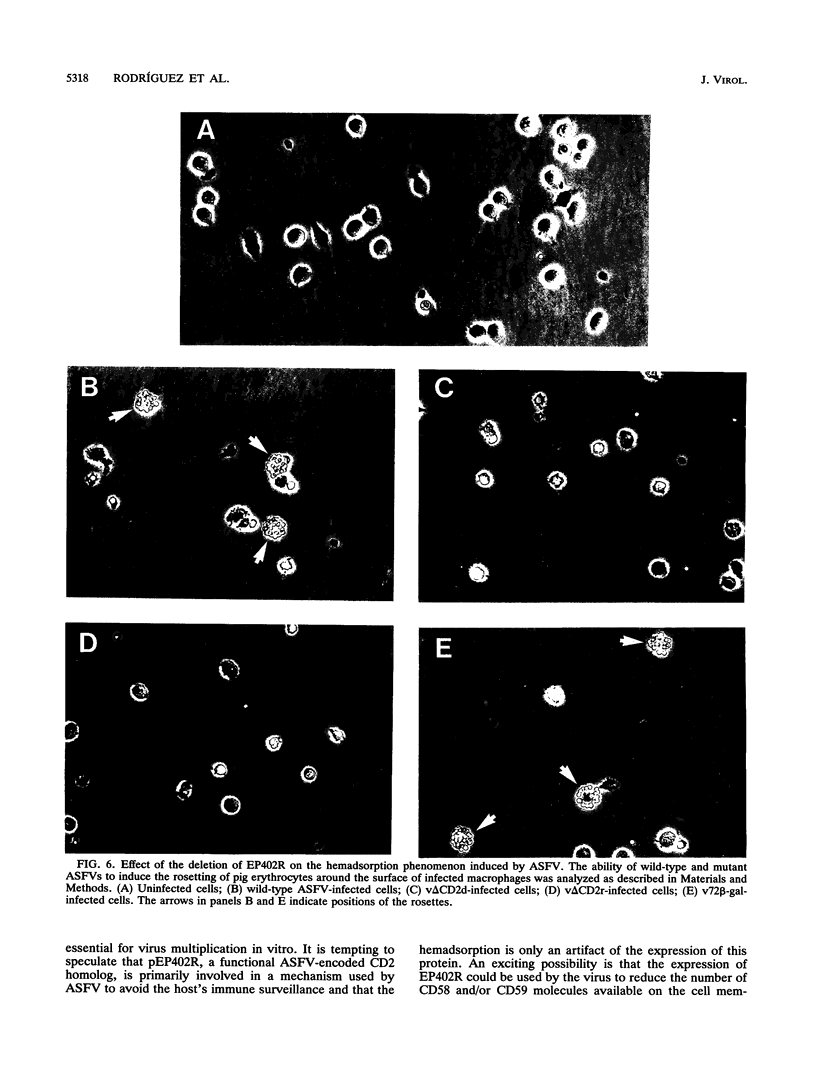
We have identified an open reading frame, EP402R, within the EcoRI E' fragment of the African swine fever virus genome that encodes a polypeptide of 402 amino acid residues homologous to the adhesion receptor of T cells, CD2. Transcription of EP402R takes place during the late phase of virus replication. The disruption of EP402R, achieved through the replacement of a 354-bp-long fragment from within EP402R by the marker gene lacZ, does not affect the virus growth rate in vitro but abrogates the ability of the virus to induce the adsorption of pig erythrocytes to the surface of infected cells. This result demonstrates that the protein encoded by EP402R is directly involved in the hemadsorption phenomenon induced by the infection of susceptible cells with African swine fever virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almazán F., Rodríguez J. M., Andrés G., Pérez R., Viñuela E., Rodriguez J. F. Transcriptional analysis of multigene family 110 of African swine fever virus. J Virol. 1992 Nov;66(11):6655–6667. doi: 10.1128/jvi.66.11.6655-6667.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazán F., Rodríguez J. M., Angulo A., Viñuela E., Rodriguez J. F. Transcriptional mapping of a late gene coding for the p12 attachment protein of African swine fever virus. J Virol. 1993 Jan;67(1):553–556. doi: 10.1128/jvi.67.1.553-556.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Beyers A. D., Barclay A. N., Law D. A., He Q., Williams A. F. Activation of T lymphocytes via monoclonal antibodies against rat cell surface antigens with particular reference to CD2 antigen. Immunol Rev. 1989 Oct;111:59–77. doi: 10.1111/j.1600-065x.1989.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Burakoff S. J. T-lymphocyte activation: the biology and function of CD2 and CD4. Immunol Rev. 1989 Oct;111:267–294. doi: 10.1111/j.1600-065x.1989.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Carrascosa A. L., Santarén J. F., Viñuela E. Production and titration of African swine fever virus in porcine alveolar macrophages. J Virol Methods. 1982 Jan;3(6):303–310. doi: 10.1016/0166-0934(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll P. C., Cyster J. G., Campbell I. D., Williams A. F. Structure of domain 1 of rat T lymphocyte CD2 antigen. Nature. 1991 Oct 24;353(6346):762–765. doi: 10.1038/353762a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Moreno M. A., Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Hahn W. C., Menu E., Bothwell A. L., Sims P. J., Bierer B. E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992 Jun 26;256(5065):1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Davis S. J., Williams A. F., Harlos K., Stuart D. I. Crystal structure at 2.8 A resolution of a soluble form of the cell adhesion molecule CD2. Nature. 1992 Nov 19;360(6401):232–239. doi: 10.1038/360232a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Ley V., Almendral J. M., Carbonero P., Beloso A., Viñuela E., Talavera A. Molecular cloning of African swine fever virus DNA. Virology. 1984 Mar;133(2):249–257. doi: 10.1016/0042-6822(84)90392-1. [DOI] [PubMed] [Google Scholar]
- MALMQUIST W. A., HAY D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960 Jan;21:104–108. [PubMed] [Google Scholar]
- Oldstone M. B. Molecular anatomy of viral persistence. J Virol. 1991 Dec;65(12):6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A., Seed B. Monoclonal antibody and ligand binding sites of the T cell erythrocyte receptor (CD2). 1987 Oct 29-Nov 4Nature. 329(6142):842–846. doi: 10.1038/329842a0. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. M., Almazán F., Viñuela E., Rodriguez J. F. Genetic manipulation of African swine fever virus: construction of recombinant viruses expressing the beta-galactosidase gene. Virology. 1992 May;188(1):67–76. doi: 10.1016/0042-6822(92)90735-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Dunne J., Owen M. J., Crumpton M. J. Molecular cloning of the human T-lymphocyte surface CD2 (T11) antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8718–8722. doi: 10.1073/pnas.83.22.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M. A., Gomez-Villamandos J. C., Carrasco L., Fernandez A., Mozos E., Jover A. In vivo study of hemadsorption in African swine fever virus infected cells. Vet Pathol. 1991 Mar;28(2):178–181. doi: 10.1177/030098589102800213. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Whitlow M. B., Iida K., Stefanova I., Bernard A., Nussenzweig V. H19, a surface membrane molecule involved in T-cell activation, inhibits channel formation by human complement. Cell Immunol. 1990 Mar;126(1):176–184. doi: 10.1016/0008-8749(90)90310-n. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Clark S. J., Paterson D. J., Willis A. C. Similarities in sequences and cellular expression between rat CD2 and CD4 antigens. J Exp Med. 1987 Feb 1;165(2):368–380. doi: 10.1084/jem.165.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yagita H., Okumura K., Nakauchi H. Molecular cloning of the murine homologue of CD2. Homology of the molecule to its human counterpart T11. J Immunol. 1988 Feb 15;140(4):1321–1326. [PubMed] [Google Scholar]