Abstract
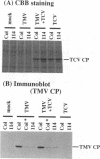
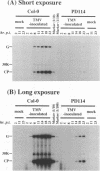
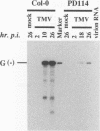
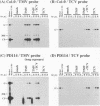
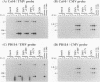
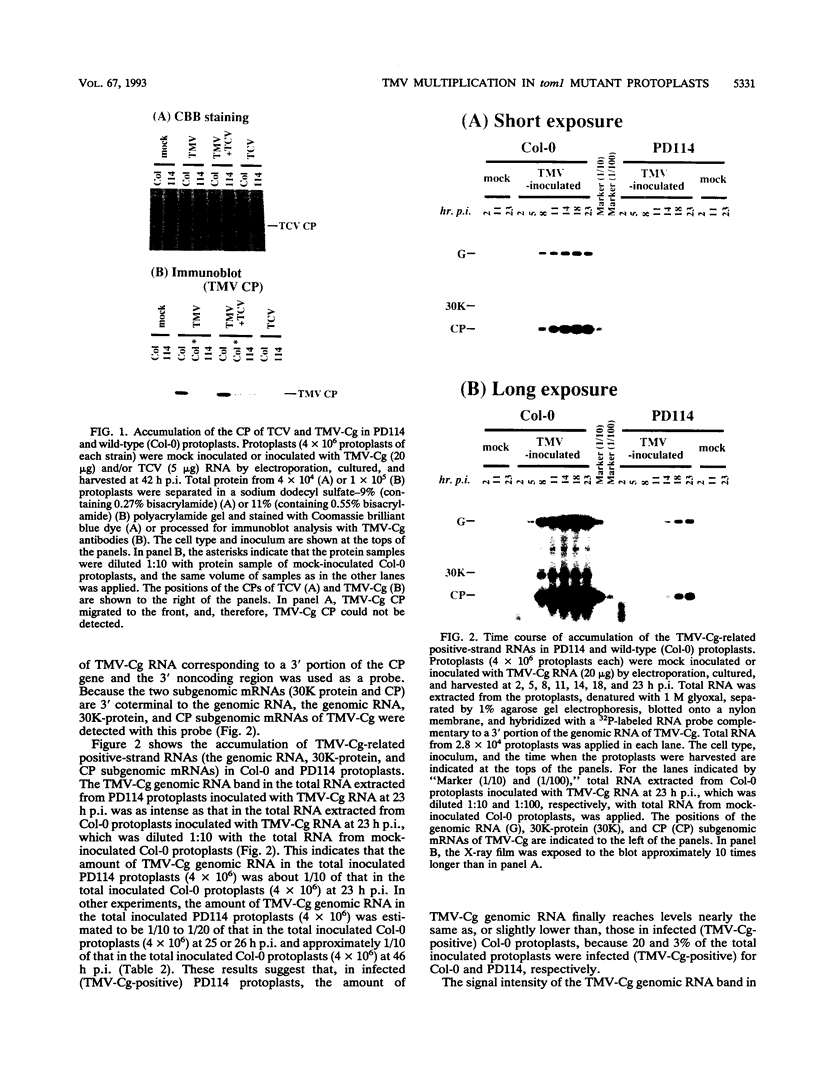
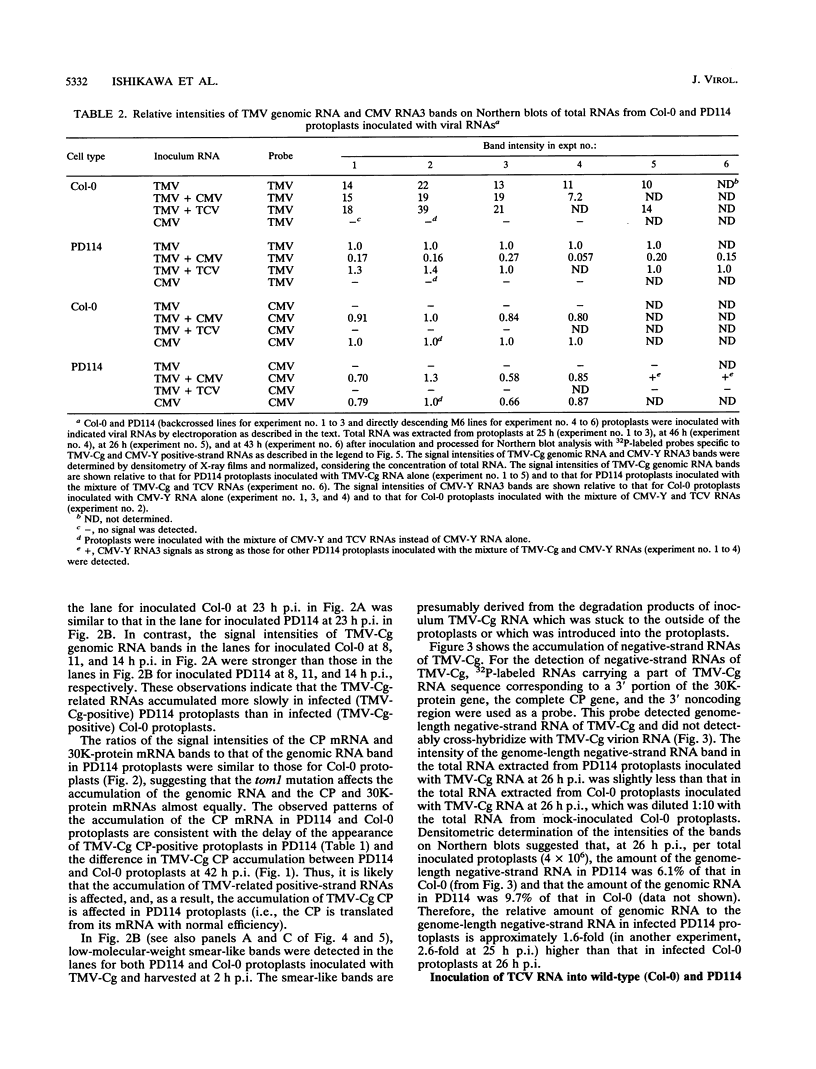
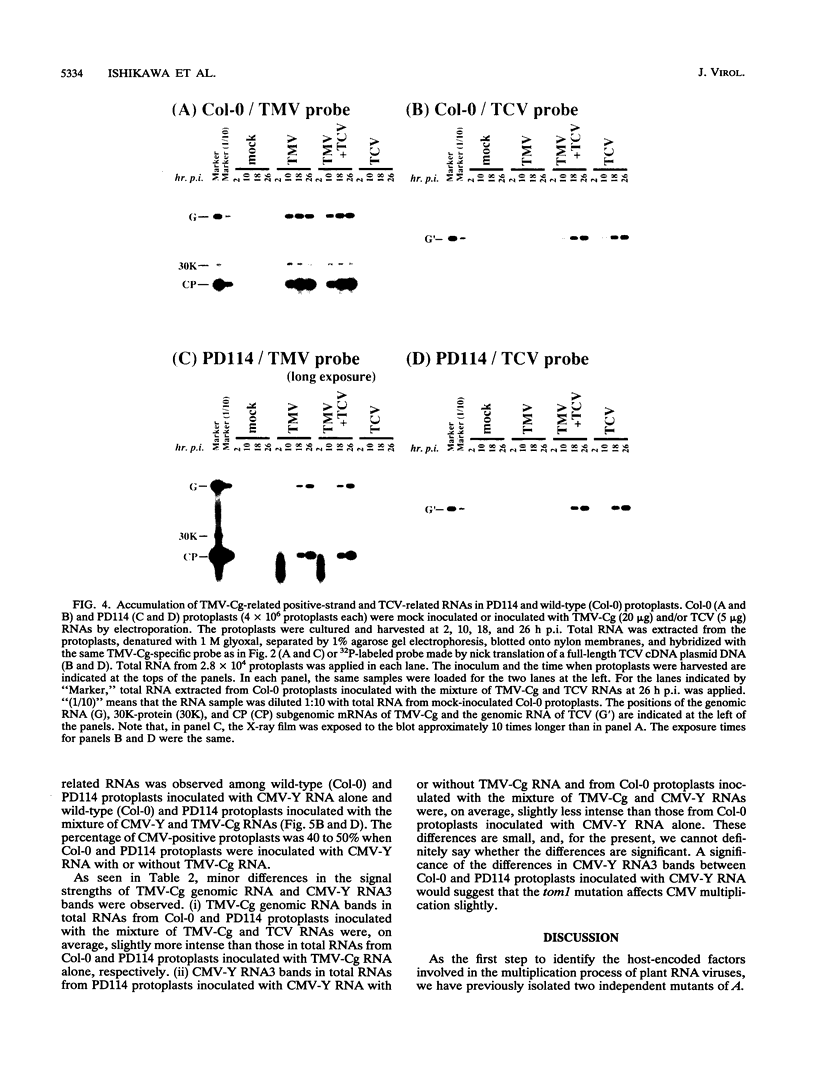
For the multiplication of RNA viruses, specific host factors are considered essential, but as of yet little is known about this aspect of virus multiplication. To identify such host factors, we previously isolated PD114, a mutant of Arabidopsis thaliana, in which the accumulation of the coat protein of tobacco mosaic virus (TMV) in uninoculated leaves of an infected plant was reduced to low levels. The causal mutation, designated tom1, was single, nuclear, and recessive. Here, we demonstrate that the tom1 mutation affects the amplification of TMV-related RNAs in a single cell. When protoplasts were inoculated with TMV RNA by electroporation, the percentage of TMV-positive protoplasts (detected by indirect immunofluorescence staining with anti-TMV antibodies) was lower (about 1/5 to 1/10) among PD114 protoplasts than among wild-type protoplasts. In TMV-positive PD114 protoplasts, the amounts of the positive-strand RNAs (the genomic RNA and subgenomic mRNAs) and coat protein reached levels similar to, or slightly lower than, those reached in TMV-positive wild-type protoplasts, but the accumulation of the positive-strand RNAs and coat protein occurred more slowly than with the wild-type protoplasts. The parallel decrease in the amounts of the coat protein and its mRNA suggests that the coat protein is translated from its mRNA with normal efficiency. These observations support the idea that the TOM1 gene encodes a host factor necessary for the efficient amplification of TMV RNA in an infected cell. Furthermore, we show that TMV multiplication in PD114 protoplasts is severely affected by the coinoculation of cucumber mosaic virus (CMV) RNA. When PD114 protoplasts were inoculated with a mixture of TMV and CMV RNAs by electroporation, the accumulation of TMV-related molecules was approximately one-fifth of that in PD114 protoplasts inoculated with TMV RNA alone. No such reduction in the accumulation of TMV-related molecules was observed when wild-type protoplasts were inoculated with a mixture of TMV and CMV RNAs or when wild-type and PD114 protoplasts were inoculated with a mixture of TMV and turnip crinkle virus RNAs. These observations are compatible with a hypothetical model in which a gene(s) that is distinct from the TOM1 gene is involved in both TMV and CMV multiplication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Oliver M. J., Beachy R. N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987 Jul 24;237(4813):389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Hauge B. M., Valon C., Smalle J., Parcy F., Goodman H. M. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992 Oct;4(10):1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P., Ecker J. R. Development of large DNA methods for plants: molecular cloning of large segments of Arabidopsis and carrot DNA into yeast. Nucleic Acids Res. 1988 Dec 9;16(23):11091–11105. doi: 10.1093/nar/16.23.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Kroner P., Ahlquist P., Meshi T. Biological activities of hybrid RNAs generated by 3'-end exchanges between tobacco mosaic and brome mosaic viruses. J Virol. 1991 Jul;65(7):3451–3459. doi: 10.1128/jvi.65.7.3451-3459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Motoyoshi F., Takamatsu N., Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986 Nov 11;14(21):8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y. Specific cessation of minus-strand RNA accumulation at an early stage of tobacco mosaic virus infection. J Virol. 1991 Feb;65(2):861–868. doi: 10.1128/jvi.65.2.861-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Obata F., Kumagai T., Ohno T. Isolation of mutants of Arabidopsis thaliana in which accumulation of tobacco mosaic virus coat protein is reduced to low levels. Mol Gen Genet. 1991 Nov;230(1-2):33–38. doi: 10.1007/BF00290647. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Watanabe Y., Saito T., Sugimoto A., Maeda T., Okada Y. Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987 Sep;6(9):2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M. Arabidopsis, a useful weed. Cell. 1989 Jan 27;56(2):263–269. doi: 10.1016/0092-8674(89)90900-8. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Pruitt R. E. Arabidopsis thaliana and Plant Molecular Genetics. Science. 1985 Sep 20;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Boyer J. C., Haenni A. L. Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6157–6173. doi: 10.1093/nar/16.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Aoyagi M., Yamanashi Y., Saito H., Ikawa S., Meshi T., Okada Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J Biochem. 1984 Dec;96(6):1915–1923. doi: 10.1093/oxfordjournals.jbchem.a135026. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I. Fluorescent antibody staining of tobacco mosaic virus antigen in tobacco mesophyll protoplasts. Virology. 1969 Jul;38(3):497–499. doi: 10.1016/0042-6822(69)90167-6. [DOI] [PubMed] [Google Scholar]
- Pardigon N., Strauss J. H. Cellular proteins bind to the 3' end of Sindbis virus minus-strand RNA. J Virol. 1992 Feb;66(2):1007–1015. doi: 10.1128/jvi.66.2.1007-1015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Symons R. H. Cucumber mosaic virus contains a functionally divided genome. Virology. 1973 Jun;53(2):487–492. doi: 10.1016/0042-6822(73)90232-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Quadt R., Kao C. C., Browning K. S., Hershberger R. P., Ahlquist P. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1498–1502. doi: 10.1073/pnas.90.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Yamanaka K., Okada Y. Long-distance movement and viral assembly of tobacco mosaic virus mutants. Virology. 1990 Jun;176(2):329–336. doi: 10.1016/0042-6822(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Sun Tp., Goodman H. M., Ausubel F. M. Cloning the Arabidopsis GA1 Locus by Genomic Subtraction. Plant Cell. 1992 Feb;4(2):119–128. doi: 10.1105/tpc.4.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Watanabe Y., Meshi T., Okada Y. The initiation site for transcription of the TMV 30-kDa protein messenger RNA. FEBS Lett. 1984 Jul 23;173(1):247–250. doi: 10.1016/0014-5793(84)81056-x. [DOI] [PubMed] [Google Scholar]
- Weigel D., Alvarez J., Smyth D. R., Yanofsky M. F., Meyerowitz E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992 May 29;69(5):843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- Yanofsky M. F., Ma H., Bowman J. L., Drews G. N., Feldmann K. A., Meyerowitz E. M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990 Jul 5;346(6279):35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]