Abstract
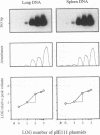
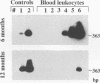
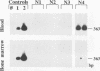
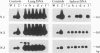
Recurrence of infectious virus from the latent viral genomes is the initiating event in the pathogenesis of cytomegalovirus (CMV) disease during states of immunodeficiency. Interstitial pneumonia is a frequent manifestation of posttransplantation CMV disease, in particular after bone marrow transplantation and heart and lung transplantations. Recurrence can occur within the transplant derived from a latent infected donor as well as within latently infected organs of the transplant recipient. The reason for a predilection of the lungs as a site of CMV pathology is so far unknown. In a murine model of CMV latency, the lungs were identified as an authentic site of latent infection, since the viral genome remained detectable in lung tissue even after it was cleared to an undetectable level in blood and bone marrow. A comparison between the lungs and the spleen, the previously most thoroughly investigated site of murine CMV latency, revealed a 10-fold-higher burden of latent viral genome for the lungs. Most important, the organ-specific risk of in vivo recurrence was found to correlate with the organ-specific viral genomic load. This new finding thus characterizes the lungs as a high-risk organ for CMV recurrence, and this fact may explain in part why interstitial pneumonia is a frequent manifestation of recurrent CMV infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Brautigam A. R., Dutko F. J., Olding L. B., Oldstone M. B. Pathogenesis of murine cytomegalovirus infection: the macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J Gen Virol. 1979 Aug;44(2):349–359. doi: 10.1099/0022-1317-44-2-349. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Pass R. F., Stagno S., Alford C. A. Pediatric cytomegalovirus infection. Transplant Proc. 1991 Jun;23(3 Suppl 3):115–117. [PubMed] [Google Scholar]
- Buckley P. J., Dickson S. A., Walker W. S. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985 Apr;134(4):2310–2315. [PubMed] [Google Scholar]
- Busch F. W., Mutter W., Koszinowski U. H., Reddehase M. J. Rescue of myeloid lineage-committed preprogenitor cells from cytomegalovirus-infected bone marrow stroma. J Virol. 1991 Feb;65(2):981–984. doi: 10.1128/jvi.65.2.981-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler B., Keil G. M., Weiland F., Koszinowski U. H. Characterization of the murine cytomegalovirus early transcription unit e1 that is induced by immediate-early proteins. J Virol. 1990 May;64(5):1907–1919. doi: 10.1128/jvi.64.5.1907-1919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Lang D. J. Detection of latent cytomegalovirus in murine salivary and prostate explant cultures and cells. Infect Immun. 1977 Feb;15(2):568–575. doi: 10.1128/iai.15.2.568-574.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A., Keil G. M., Knust E., Koszinowski U. H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983 Sep;47(3):421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S. J. Clinical insights and observations contributing to the understanding of cytomegalovirus disease pathogenesis. Bone marrow transplantation. Transplant Proc. 1991 Jun;23(3 Suppl 3):110-4, discussion 114. [PubMed] [Google Scholar]
- Hamilton J. D., Seaworth B. J. Transmission of latent cytomegalovirus in a murine kidney tissue transplantation model. Transplantation. 1985 Mar;39(3):290–296. doi: 10.1097/00007890-198503000-00017. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S. Cytomegalovirus and its role in the pathogenesis of acquired immunodeficiency syndrome. Transplant Proc. 1991 Jun;23(3 Suppl 3):118-21, discussion 121. [PubMed] [Google Scholar]
- Ho M. Observations from transplantation contributing to the understanding of pathogenesis of CMV infection. Transplant Proc. 1991 Jun;23(3 Suppl 3):104-8, discussion 108-9. [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976 Jul 1;72(1):235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65(12):6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C. Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infect Immun. 1978 Jul;21(1):275–280. doi: 10.1128/iai.21.1.275-280.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C. Latent infection and the elusive cytomegalovirus. Rev Infect Dis. 1983 Mar-Apr;5(2):205–215. doi: 10.1093/clinids/5.2.205. [DOI] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman M. E., Henry S. C., Greene R. C., Brazy P. C., Klotman P. E., Hamilton J. D. Detection of mouse cytomegalovirus nucleic acid in latently infected mice by in vitro enzymatic amplification. J Infect Dis. 1990 Feb;161(2):220–225. doi: 10.1093/infdis/161.2.220. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Spector D. H. Replication of the murine cytomegalovirus genome: structure and role of the termini in the generation and cleavage of concatenates. Virology. 1988 Jan;162(1):98–107. doi: 10.1016/0042-6822(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Wiley C. A., Spector D. H. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol. 1988 Mar;62(3):987–997. doi: 10.1128/jvi.62.3.987-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerle M., Bühler B., Keil G. M., Koszinowski U. H. Structural organization, expression, and functional characterization of the murine cytomegalovirus immediate-early gene 3. J Virol. 1992 Jan;66(1):27–36. doi: 10.1128/jvi.66.1.27-36.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D., Flournoy N., Thomas E. D. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986 Mar;153(3):478–488. doi: 10.1093/infdis/153.3.478. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Jr, Abenes G. B., Manning W. C., Sambucetti L. C., Cherrington J. M. Molecular genetic analysis of cytomegalovirus gene regulation in growth, persistence and latency. Curr Top Microbiol Immunol. 1990;154:47–74. doi: 10.1007/978-3-642-74980-3_3. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy C., Hilleren P. J., Jordan M. C. Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J Virol. 1991 Jun;65(6):3330–3334. doi: 10.1128/jvi.65.6.3330-3334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Roizmann B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Wilson E. J., Barrett L. V., Medearis D. N. Primary cytomegalovirus infection following cardiac transplantation in a murine model. Transplantation. 1984 Mar;37(3):306–310. doi: 10.1097/00007890-198403000-00018. [DOI] [PubMed] [Google Scholar]
- SMITH M. G. Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med. 1954 Jul;86(3):435–440. doi: 10.3181/00379727-86-21123. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scalzo A. A., Fitzgerald N. A., Simmons A., La Vista A. B., Shellam G. R. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med. 1990 May 1;171(5):1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Shanley J. D. Murine models of cytomegalovirus-associated pneumonitis. Transplant Proc. 1991 Jun;23(3 Suppl 3):12-6, discussion 16. [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Weller T. H. Pathogenesis of human cytomegalovirus-associated diseases. Historical perspective. Transplant Proc. 1991 Jun;23(3 Suppl 3):5-6, discussion 6-7. [PubMed] [Google Scholar]
- Wilson E. J., Medearis D. N., Jr, Barrett L. V., Rubin R. H. Activation of latent murine cytomegalovirus in cardiac explant and cell cultures. J Infect Dis. 1985 Sep;152(3):625–626. doi: 10.1093/infdis/152.3.625. [DOI] [PubMed] [Google Scholar]