Abstract
Foamy viruses belong to the retroviruses which possess a complex genome structure. The human foamy virus (HFV) isolate bears three open reading frames (the so-called bel genes) in the 3' region of the genome which have been reported to give rise to possibly six different proteins via alternative splicing (W. Muranyi and R. M. Flügel, J. Virol. 65:727-735, 1991). In order to analyze the requirements of these proteins for HFV replication in vitro, we constructed a set of single and combinatory bel gene mutants of an infectious molecular clone of HFV. The mutant which lacked the transacting activator, bel-1, was found to be replication incompetent. All other mutants replicated equally well and gave rise to comparable titers of infectious cell-free virus. When HFV proviruses were put under the control of a heterologous promoter (simian virus 40), none of the accessory gene products was found to be required for expression of structural (gag) proteins. There was no evidence for a posttranscriptional regulatory protein that is present in other complex retroviruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achong B. G., Mansell P. W., Epstein M. A., Clifford P. An unusual virus in cultures from a human nasopharyngeal carcinoma. J Natl Cancer Inst. 1971 Feb;46(2):299–307. [PubMed] [Google Scholar]
- Aguzzi A., Wagner E. F., Netzer K. O., Bothe K., Anhauser I., Rethwilm A. Human foamy virus proteins accumulate in neurons and induce multinucleated giant cells in the brain of transgenic mice. Am J Pathol. 1993 Apr;142(4):1061–1071. [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988 Sep 16;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Jalinoos Y., Proulx J., Sodroski J. G., Haseltine W. A. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3(1):11–18. [PubMed] [Google Scholar]
- Cullen B. R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991 Mar;65(3):1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of human immunodeficiency virus replication. Annu Rev Microbiol. 1991;45:219–250. doi: 10.1146/annurev.mi.45.100191.001251. [DOI] [PubMed] [Google Scholar]
- Ellinger S., Glockshuber R., Jahn G., Plückthun A. Cleavage of procaryotically expressed human immunodeficiency virus fusion proteins by factor Xa and application in western blot (immunoblot) assays. J Clin Microbiol. 1989 May;27(5):971–976. doi: 10.1128/jcm.27.5.971-976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Rethwilm A., Maurer B., Darai G. Nucleotide sequence analysis of the env gene and its flanking regions of the human spumaretrovirus reveals two novel genes. EMBO J. 1987 Jul;6(7):2077–2084. doi: 10.1002/j.1460-2075.1987.tb02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M. Spumaviruses: a group of complex retroviruses. J Acquir Immune Defic Syndr. 1991;4(8):739–750. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammes S. R., Dixon E. P., Malim M. H., Cullen B. R., Greene W. C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Sun J. D., Garrett E. D., Cullen B. R. Functional organization of the Bel-1 trans activator of human foamy virus. J Virol. 1993 Apr;67(4):1896–1904. doi: 10.1128/jvi.67.4.1896-1904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Partin K. M., Löchelt M., Bannert H., Flügel R. M., Cullen B. R. Characterization of the transcriptional trans activator of human foamy retrovirus. J Virol. 1991 May;65(5):2589–2594. doi: 10.1128/jvi.65.5.2589-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kim S., Ikeuchi K., Byrn R., Groopman J., Baltimore D. Lack of a negative influence on viral growth by the nef gene of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9544–9548. doi: 10.1073/pnas.86.23.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Müller J., Müller-Hermelink H., Fuchs D., Wachter H., Daniel M. M. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993 Feb;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J., Lee A. H., Sung Y. C. Multiple positive and negative cis-acting elements that mediate transactivation by bel1 in the long terminal repeat of human foamy virus. J Virol. 1993 Apr;67(4):2317–2326. doi: 10.1128/jvi.67.4.2317-2326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löchelt M., Zentgraf H., Flügel R. M. Construction of an infectious DNA clone of the full-length human spumaretrovirus genome and mutagenesis of the bel 1 gene. Virology. 1991 Sep;184(1):43–54. doi: 10.1016/0042-6822(91)90820-2. [DOI] [PubMed] [Google Scholar]
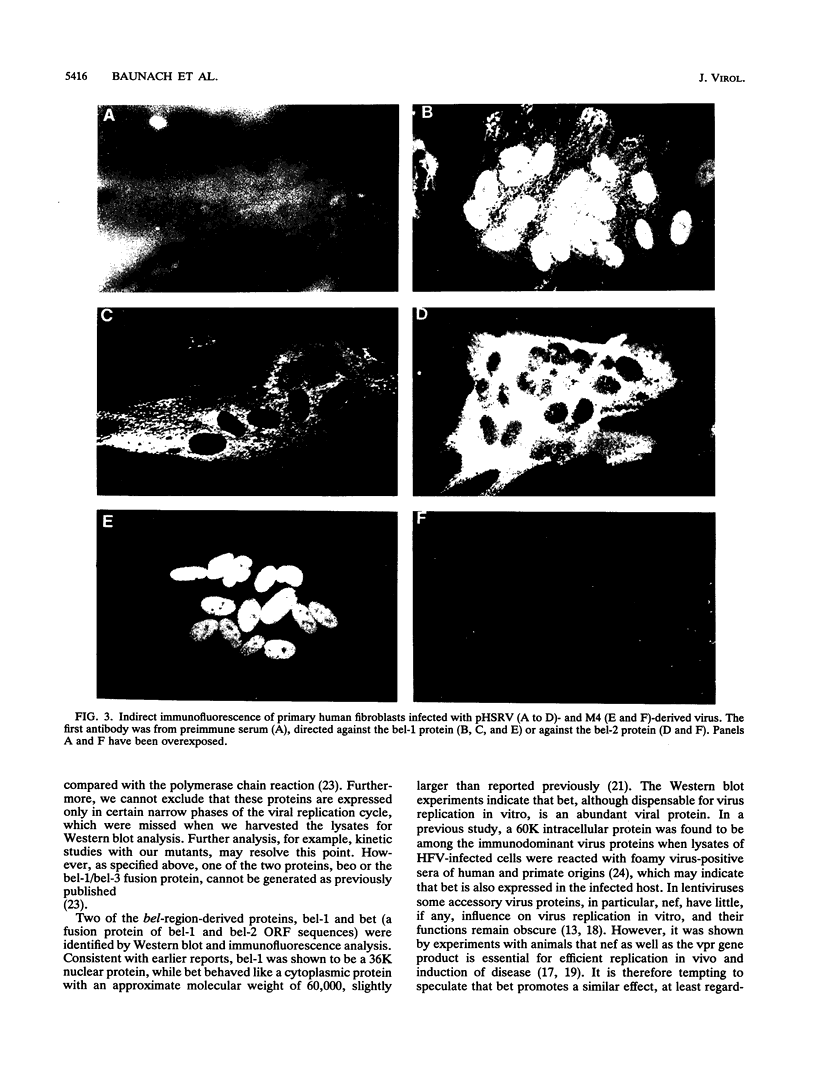
- Muranyi W., Flügel R. M. Analysis of splicing patterns of human spumaretrovirus by polymerase chain reaction reveals complex RNA structures. J Virol. 1991 Feb;65(2):727–735. doi: 10.1128/jvi.65.2.727-735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer K. O., Rethwilm A., Maurer B., ter Meulen V. Identification of the major immunogenic structural proteins of human foamy virus. J Gen Virol. 1990 May;71(Pt 5):1237–1241. doi: 10.1099/0022-1317-71-5-1237. [DOI] [PubMed] [Google Scholar]
- Netzer K. O., Schliephake A., Maurer B., Watanabe R., Aguzzi A., Rethwilm A. Identification of pol-related gene products of human foamy virus. Virology. 1993 Jan;192(1):336–338. doi: 10.1006/viro.1993.1039. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin D., Rethwilm A., Bauer G., Gudat F., zur Hausen H. Characterization of a foamy virus isolated from Cercopithecus aethiops lymphoblastoid cells. Med Microbiol Immunol. 1983;172(2):75–86. doi: 10.1007/BF02124508. [DOI] [PubMed] [Google Scholar]
- Niederman T. M., Thielan B. J., Ratner L. Human immunodeficiency virus type 1 negative factor is a transcriptional silencer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1128–1132. doi: 10.1073/pnas.86.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Felber B. K. Regulation of expression of human immunodeficiency virus. New Biol. 1990 Jan;2(1):20–31. [PubMed] [Google Scholar]
- Rethwilm A., Baunach G., Netzer K. O., Maurer B., Borisch B., ter Meulen V. Infectious DNA of the human spumaretrovirus. Nucleic Acids Res. 1990 Feb 25;18(4):733–738. doi: 10.1093/nar/18.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethwilm A., Darai G., Rösen A., Maurer B., Flügel R. M. Molecular cloning of the genome of human spumaretrovirus. Gene. 1987;59(1):19–28. doi: 10.1016/0378-1119(87)90262-9. [DOI] [PubMed] [Google Scholar]
- Rethwilm A., Erlwein O., Baunach G., Maurer B., ter Meulen V. The transcriptional transactivator of human foamy virus maps to the bel 1 genomic region. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):941–945. doi: 10.1073/pnas.88.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Campbell M., Nasioulas G., Harrison J., Felber B. K., Pavlakis G. N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992 Dec;66(12):7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Venkatesh L. K., Yang C., Theodorakis P. A., Chinnadurai G. Functional dissection of the human spumaretrovirus transactivator identifies distinct classes of dominant-negative mutants. J Virol. 1993 Jan;67(1):161–169. doi: 10.1128/jvi.67.1.161-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]