Abstract
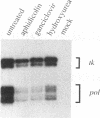
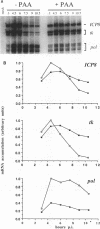
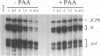
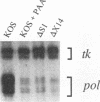
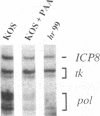
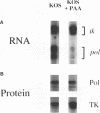
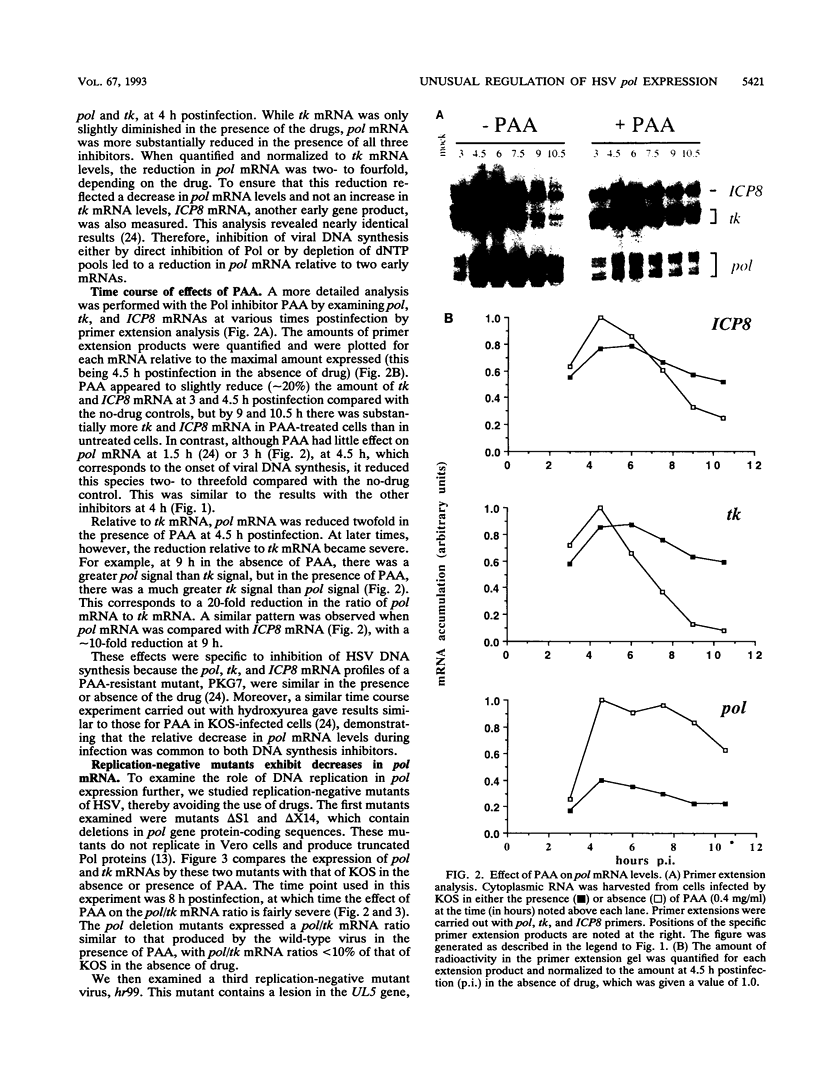
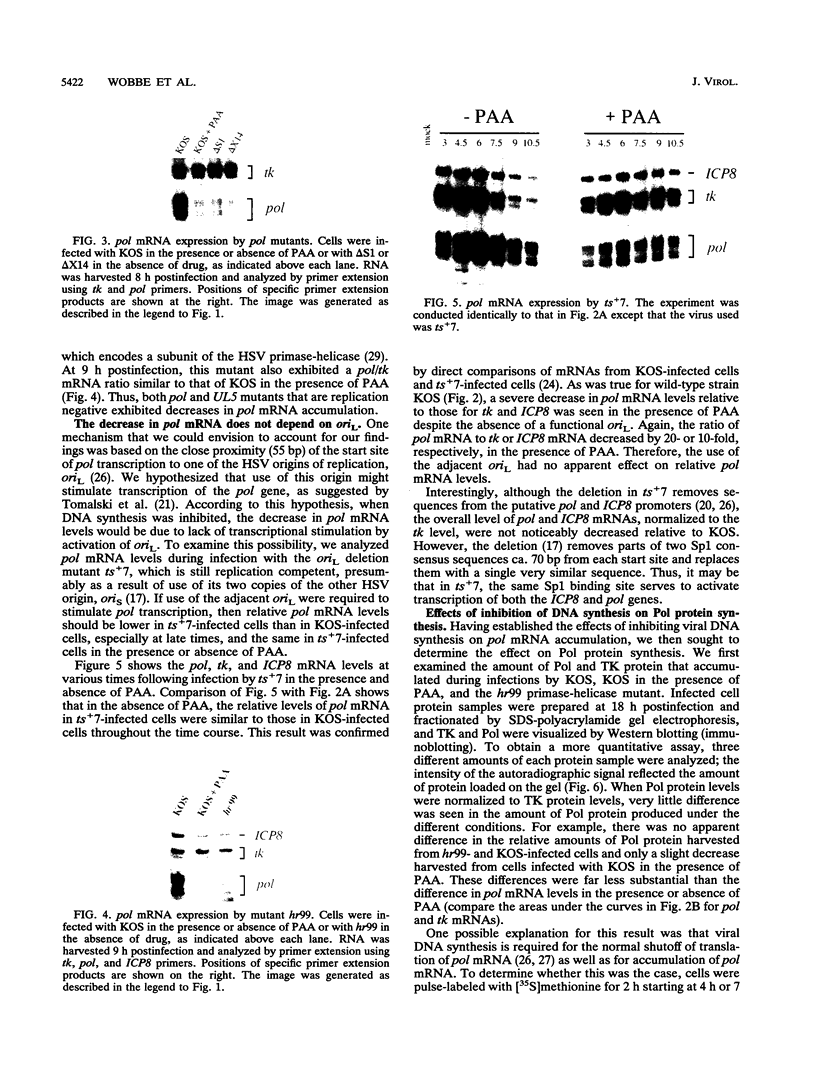
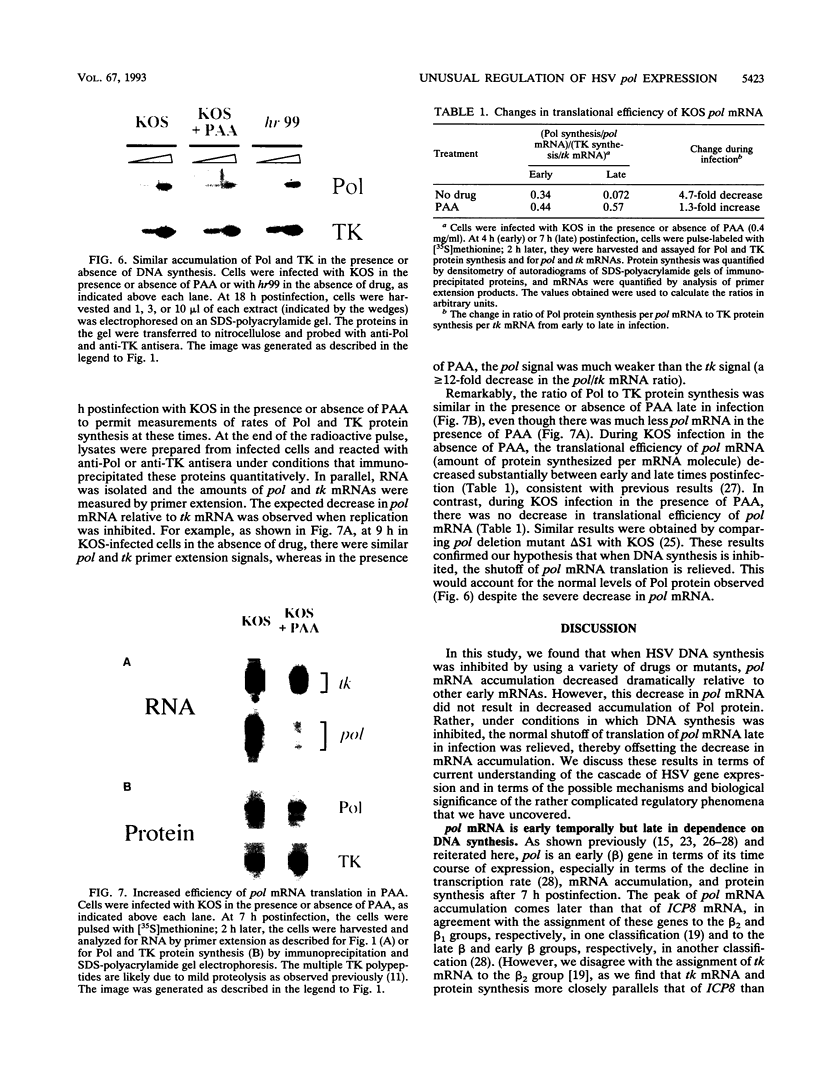
During herpes simplex virus infection, expression of the viral DNA polymerase (pol) gene is regulated temporally as an early (beta) gene and is additionally down-regulated at late times at the level of translation (D. R. Yager, A. I. Marcy, and D. M. Coen, J. Virol. 64:2217-2225, 1990). To examine the role of viral DNA synthesis in pol regulation, we studied pol expression during infections in which viral DNA synthesis was blocked, either by using drugs that inhibit Pol or ribonucleotide reductase or by using viral mutants with lesions in either the pol or a primase-helicase subunit gene. Under any of these conditions, the level of cytoplasmic pol mRNA was reduced. This reduction was first seen at approximately the time DNA synthesis begins and, when normalized to levels of other early mRNAs, became as great as 20-fold late in infection. The reduction was also observed in the absence of the adjacent origin of replication, oriL. Thus, although pol mRNA accumulated as expected for an early gene in terms of temporal regulation, it behaved more like that of a late (gamma) gene in its response to DNA synthesis inhibition. Surprisingly, despite the marked decrease in pol mRNA in the absence of DNA synthesis, the accumulation of Pol polypeptide was unaffected. This was accompanied by loss of the normal down-regulation of translation of pol mRNA at late times. We suggest a model to explain these findings.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrake M., Guild N., Hsu T., Gold L., Tuerk C., Karam J. DNA polymerase of bacteriophage T4 is an autogenous translational repressor. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7942–7946. doi: 10.1073/pnas.85.21.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau H., Freese U. K. Analysis of the HSV-1 strain 17 DNA polymerase gene reveals the expression of four different classes of pol transcripts. Virology. 1991 Aug;183(2):505–518. doi: 10.1016/0042-6822(91)90980-p. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicioccio R. A., Chadha K., Sahai Srivastava B. I. Inhibition of herpes simplex virus-induced DNA polymerase, cellular DNA polymerase alpha, and virus production by aphidicolin. Biochim Biophys Acta. 1980 Sep 19;609(2):224–231. doi: 10.1016/0005-2787(80)90233-6. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Larsson A., Helgstrand E., Johansson N. G., Oberg B. Pyrophosphate analogues as inhibitors of herpes simplex virus type 1 DNA polymerase. Biochim Biophys Acta. 1980 Mar 28;607(1):53–64. doi: 10.1016/0005-2787(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Frank K. B., Derse D. D., Bastow K. F., Cheng Y. C. Novel interaction of aphidicolin with herpes simplex virus DNA polymerase and polymerase-associated exonuclease. J Biol Chem. 1984 Nov 10;259(21):13282–13286. [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A. F., Manos M. M., Jacobson J. G., Gibbs J. S., Coen D. M. Effect of an amber mutation in the herpes simplex virus thymidine kinase gene on polypeptide synthesis and stability. Virology. 1989 Feb;168(2):210–220. doi: 10.1016/0042-6822(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Marcy A. I., Yager D. R., Coen D. M. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J Virol. 1990 May;64(5):2208–2216. doi: 10.1128/jvi.64.5.2208-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Grill S. P., Cheng Y. C. Can ribonucleotide reductase be considered as an effective target for developing antiherpes simplex virus type II (HSV-2) compounds? Biochem Pharmacol. 1985 Mar 15;34(6):777–780. doi: 10.1016/0006-2952(85)90757-9. [DOI] [PubMed] [Google Scholar]
- Oroskar A. A., Read G. S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989 May;63(5):1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvino-Bodnar M., Orberg P. K., Schaffer P. A. Herpes simplex virus type 1 oriL is not required for virus replication or for the establishment and reactivation of latent infection in mice. J Virol. 1987 Nov;61(11):3528–3535. doi: 10.1128/jvi.61.11.3528-3535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R., Mar E. C., Huang E. S., Topal M. D. Insertion and extension of acyclic, dideoxy, and ara nucleotides by herpesviridae, human alpha and human beta polymerases. A unique inhibition mechanism for 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1988 Mar 15;263(8):3898–3904. [PubMed] [Google Scholar]
- Su L., Knipe D. M. Mapping of the transcriptional initiation site of the herpes simplex virus type 1 ICP8 gene in infected and transfected cells. J Virol. 1987 Feb;61(2):615–620. doi: 10.1128/jvi.61.2.615-620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski M. D., Wu J. G., Miller L. K. The location, sequence, transcription, and regulation of a baculovirus DNA polymerase gene. Virology. 1988 Dec;167(2):591–600. [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Yager D. R., Coen D. M. Analysis of the transcript of the herpes simplex virus DNA polymerase gene provides evidence that polymerase expression is inefficient at the level of translation. J Virol. 1988 Jun;62(6):2007–2015. doi: 10.1128/jvi.62.6.2007-2015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager D. R., Marcy A. I., Coen D. M. Translational regulation of herpes simplex virus DNA polymerase. J Virol. 1990 May;64(5):2217–2225. doi: 10.1128/jvi.64.5.2217-2225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Wagner E. K. The kinetics of expression of individual herpes simplex virus type 1 transcripts. Virus Genes. 1987 Nov;1(1):49–60. doi: 10.1007/BF00125685. [DOI] [PubMed] [Google Scholar]
- Zhu L. A., Weller S. K. The UL5 gene of herpes simplex virus type 1: isolation of a lacZ insertion mutant and association of the UL5 gene product with other members of the helicase-primase complex. J Virol. 1992 Jan;66(1):458–468. doi: 10.1128/jvi.66.1.458-468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]