Abstract
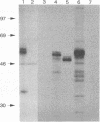
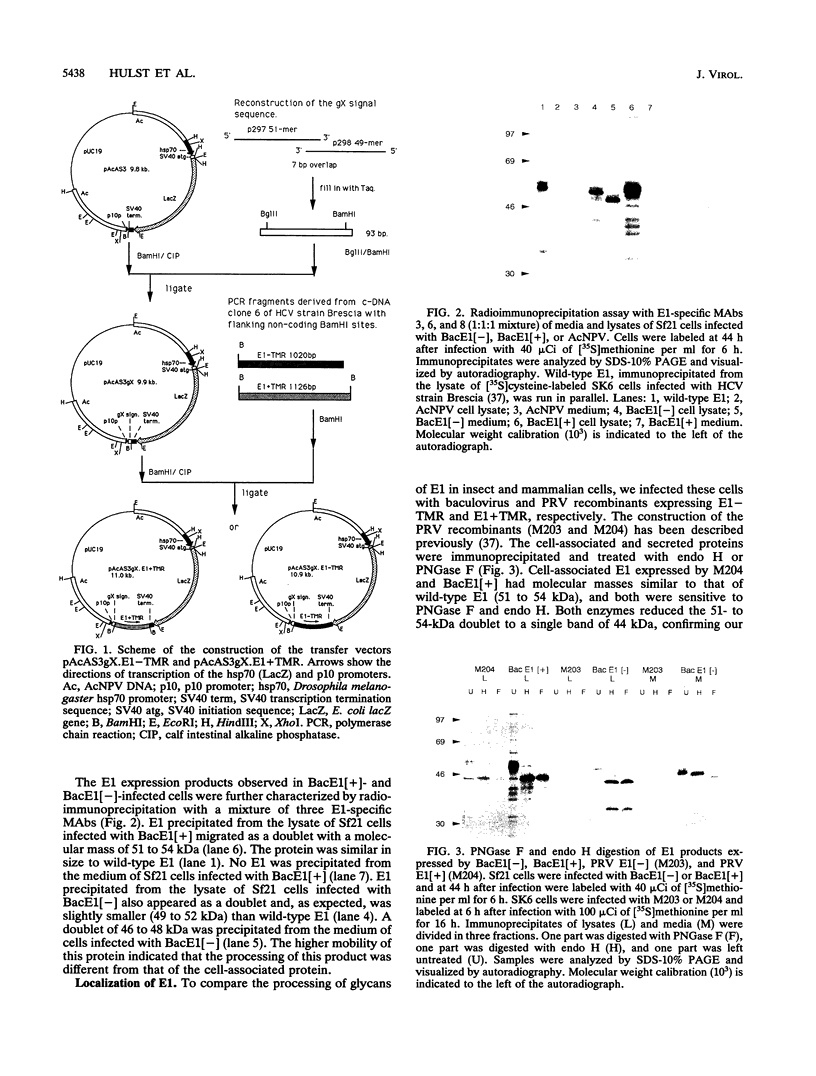
The processing and protective capacity of E1, an envelope glycoprotein of hog cholera virus (HCV), were investigated after expression of different versions of the protein in insect cells by using a baculovirus vector. Recombinant virus BacE1[+] expressed E1, including its C-terminal transmembrane region (TMR), and generated a protein which was similar in size (51 to 54 kDa) to the size of E1 expressed in swine kidney cells infected with HCV. The protein was not secreted from the insect cells, and like wild-type E1, it remained sensitive to endo-beta-N-acetyl-D-glucosaminidase H (endo H). This indicates that E1 with a TMR accumulates in the endoplasmic reticulum or cis-Golgi region of the cell. In contrast, recombinant virus BacE1[-], which expressed E1 without a C-terminal TMR, generated a protein that was secreted from the cells. The fraction of this protein that was found to be cell associated had a slightly lower molecular mass (49 to 52 kDa) than wild-type E1 and remained endo H sensitive. The high-mannose units of the secreted protein were trimmed during transport through the exocytotic pathway to endo H-resistant glycans, resulting in a protein with a lower molecular mass (46 to 48 kDa). Secreted E1 accumulated in the medium to about 30 micrograms/10(6) cells. This amount was about 3-fold higher than that of cell-associated E1 in BacE1[-] and 10-fold higher than that of cell-associated E1 in BacE1[+]-infected Sf21 cells. Intramuscular vaccination of pigs with immunoaffinity-purified E1 in a double water-oil emulsion elicited high titers of neutralizing antibodies between 2 and 4 weeks after vaccination at the lowest dose tested (20 micrograms). The vaccinated pigs were completely protected against intranasal challenge with 100 50% lethal doses of HCV strain Brescia, indicating that E1 expressed in insect cells is an excellent candidate for development of a new, safe, and effective HCV subunit vaccine.
Full text
PDF


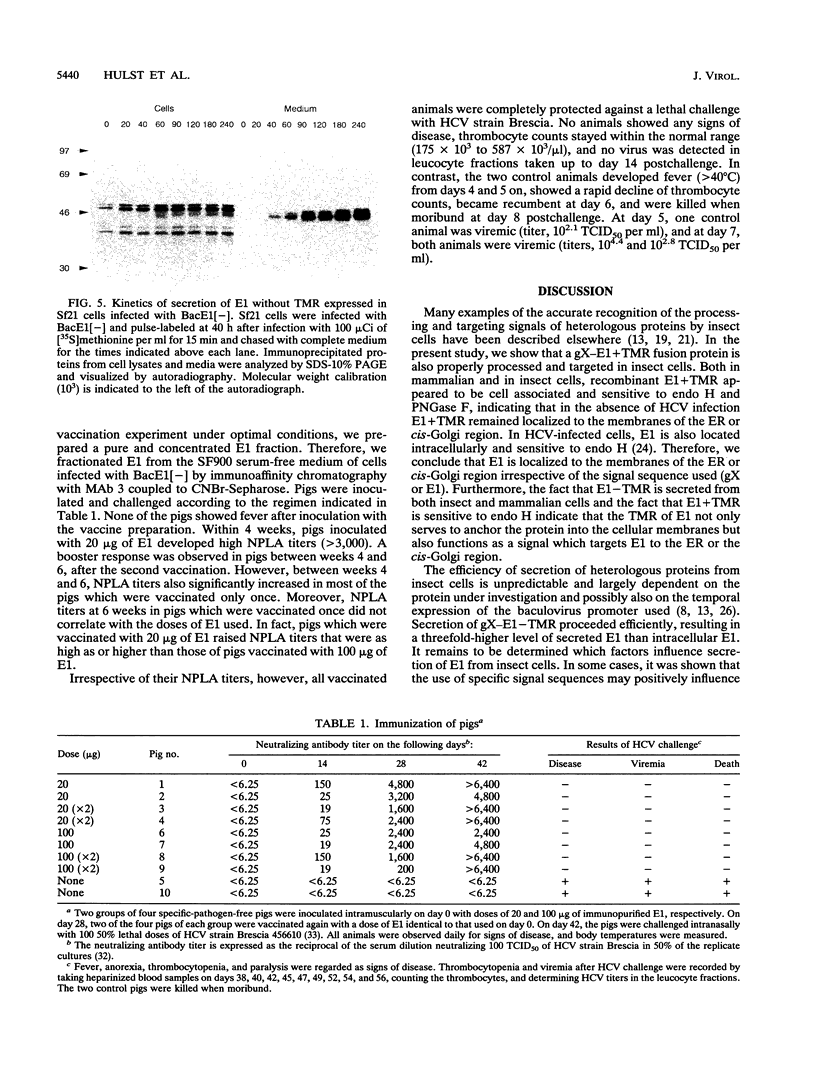


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barteling S. J., Vreeswijk J. Developments in foot-and-mouth disease vaccines. Vaccine. 1991 Feb;9(2):75–88. doi: 10.1016/0264-410x(91)90261-4. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- GRACE T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962 Aug 25;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Godet M., Rasschaert D., Laude H. Processing and antigenicity of entire and anchor-free spike glycoprotein S of coronavirus TGEV expressed by recombinant baculovirus. Virology. 1991 Dec;185(2):732–740. doi: 10.1016/0042-6822(91)90544-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert W. J. Multiple emulsions. A new form of mineral-oil antigen adjuvant. Lancet. 1965 Oct 16;2(7416):771–771. doi: 10.1016/s0140-6736(65)90816-0. [DOI] [PubMed] [Google Scholar]
- Hill-Perkins M. S., Possee R. D. A baculovirus expression vector derived from the basic protein promoter of Autographa californica nuclear polyhedrosis virus. J Gen Virol. 1990 Apr;71(Pt 4):971–976. doi: 10.1099/0022-1317-71-4-971. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Kasza L., Shadduck J. A., Christofinis G. J. Establishment, viral susceptibility and biological characteristics of a swine kidney cell line SK-6. Res Vet Sci. 1972 Jan;13(1):46–51. [PubMed] [Google Scholar]
- King L. A., Kaur K., Mann S. G., Lawrie A. M., Steven J., Ogden J. E. Secretion of single-chain urokinase-type plasminogen activator from insect cells. Gene. 1991 Oct 15;106(2):151–157. doi: 10.1016/0378-1119(91)90194-g. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Geyer H., Geyer R., Doerfler W., Klenk H. D. The oligosaccharides of influenza virus hemagglutinin expressed in insect cells by a baculovirus vector. Virology. 1990 Feb;174(2):418–429. doi: 10.1016/0042-6822(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Kwang J., Littledike E. T., Donis R. O., Dubovi E. J. Recombinant polypeptide from the gp48 region of the bovine viral diarrhea virus (BVDV) detects serum antibodies in vaccinated and infected cattle. Vet Microbiol. 1992 Oct;32(3-4):281–292. doi: 10.1016/0378-1135(92)90151-i. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem. 1988 Feb 15;263(5):2107–2110. [PubMed] [Google Scholar]
- López de Turiso J. A., Cortés E., Martínez C., Ruiz de Ybáez R., Simarro I., Vela C., Casal I. Recombinant vaccine for canine parvovirus in dogs. J Virol. 1992 May;66(5):2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., Trimble R. B., Tarentino A. L., Plummer T. H., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989 Aug 1;180(2):195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., Hulst M. M. Hog cholera virus: identification and characterization of the viral RNA and the virus-specific RNA synthesized in infected swine kidney cells. Virus Res. 1988 Nov;11(4):281–291. doi: 10.1016/0168-1702(88)90002-0. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., Warmerdam P. A., van der Meer B., Schaaper W. M., Wensvoort G., Hulst M. M. Molecular cloning and nucleotide sequence of hog cholera virus strain Brescia and mapping of the genomic region encoding envelope protein E1. Virology. 1990 Jul;177(1):184–198. doi: 10.1016/0042-6822(90)90472-4. [DOI] [PubMed] [Google Scholar]
- Noteborn M. H., de Boer G. F., Kant A., Koch G., Bos J. L., Zantema A., van der Eb A. J. Expression of avian leukaemia virus env-gp85 in Spodoptera frugiperda cells by use of a baculovirus expression vector. J Gen Virol. 1990 Nov;71(Pt 11):2641–2648. doi: 10.1099/0022-1317-71-11-2641. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliki J. T., Mizak B., Flore H. P., Gettig R. R., Burand J. P., Carmichael L. E., Wood H. A., Parrish C. R. Canine parvovirus empty capsids produced by expression in a baculovirus vector: use in analysis of viral properties and immunization of dogs. J Gen Virol. 1992 Feb;73(Pt 2):369–374. doi: 10.1099/0022-1317-73-2-369. [DOI] [PubMed] [Google Scholar]
- Terpstra C., Bloemraad M., Gielkens A. L. The neutralizing peroxidase-linked assay for detection of antibody against swine fever virus. Vet Microbiol. 1984 Apr;9(2):113–120. doi: 10.1016/0378-1135(84)90026-9. [DOI] [PubMed] [Google Scholar]
- Terpstra C., Wensvoort G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol. 1988 Feb;16(2):123–128. doi: 10.1016/0378-1135(88)90036-3. [DOI] [PubMed] [Google Scholar]
- Tessier D. C., Thomas D. Y., Khouri H. E., Laliberté F., Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991 Feb 15;98(2):177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Thiel H. J., Stark R., Weiland E., Rümenapf T., Meyers G. Hog cholera virus: molecular composition of virions from a pestivirus. J Virol. 1991 Sep;65(9):4705–4712. doi: 10.1128/jvi.65.9.4705-4712.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Vlak J. M., Keus R. J. Baculovirus expression vector system for production of viral vaccines. Adv Biotechnol Processes. 1990;14:91–128. [PubMed] [Google Scholar]
- Vlak J. M., Schouten A., Usmany M., Belsham G. J., Klinge-Roode E. C., Maule A. J., Van Lent J. W., Zuidema D. Expression of cauliflower mosaic virus gene I using a baculovirus vector based upon the p10 gene and a novel selection method. Virology. 1990 Nov;179(1):312–320. doi: 10.1016/0042-6822(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Weiland E., Stark R., Haas B., Rümenapf T., Meyers G., Thiel H. J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J Virol. 1990 Aug;64(8):3563–3569. doi: 10.1128/jvi.64.8.3563-3569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Compans R. W. Expression and characterization of a functional human immunodeficiency virus envelope glycoprotein in insect cells. Virology. 1990 Jun;176(2):575–586. doi: 10.1016/0042-6822(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Bloemraad M., Terpstra C. An enzyme immunoassay employing monoclonal antibodies and detecting specifically antibodies to classical swine fever virus. Vet Microbiol. 1988 Jun;17(2):129–140. doi: 10.1016/0378-1135(88)90004-1. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Boonstra J., Bodzinga B. G. Immunoaffinity purification and characterization of the envelope protein E1 of hog cholera virus. J Gen Virol. 1990 Mar;71(Pt 3):531–540. doi: 10.1099/0022-1317-71-3-531. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Boonstra J., Bloemraad M., Van Zaane D. Production of monoclonal antibodies against swine fever virus and their use in laboratory diagnosis. Vet Microbiol. 1986 Jul;12(2):101–108. doi: 10.1016/0378-1135(86)90072-6. [DOI] [PubMed] [Google Scholar]
- Wensvoort G. Topographical and functional mapping of epitopes on hog cholera virus with monoclonal antibodies. J Gen Virol. 1989 Nov;70(Pt 11):2865–2876. doi: 10.1099/0022-1317-70-11-2865. [DOI] [PubMed] [Google Scholar]
- van Rijn P. A., van Gennip R. G., de Meijer E. J., Moormann R. J. A preliminary map of epitopes on envelope glycoprotein E1 of HCV strain Brescia. Vet Microbiol. 1992 Nov;33(1-4):221–230. doi: 10.1016/0378-1135(92)90050-4. [DOI] [PubMed] [Google Scholar]
- van Zijl M., Wensvoort G., de Kluyver E., Hulst M., van der Gulden H., Gielkens A., Berns A., Moormann R. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol. 1991 May;65(5):2761–2765. doi: 10.1128/jvi.65.5.2761-2765.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]