Abstract
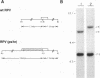
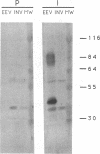
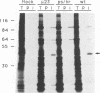
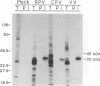
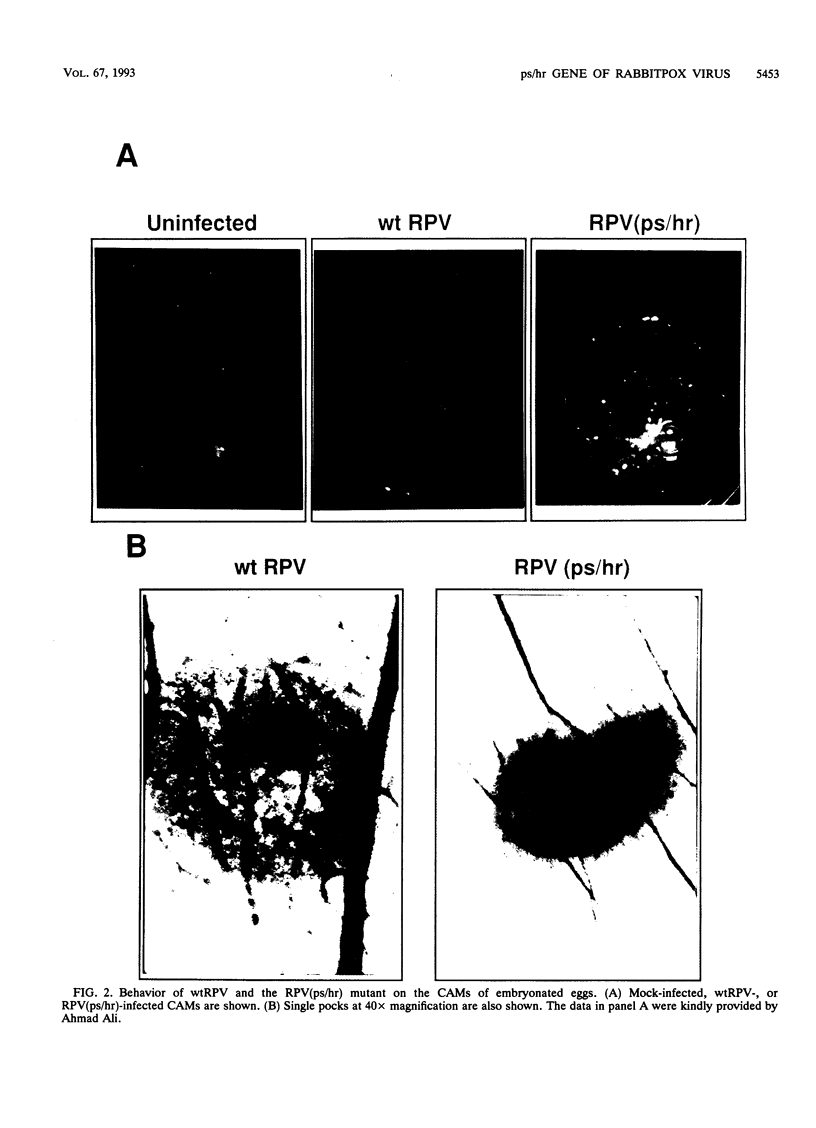
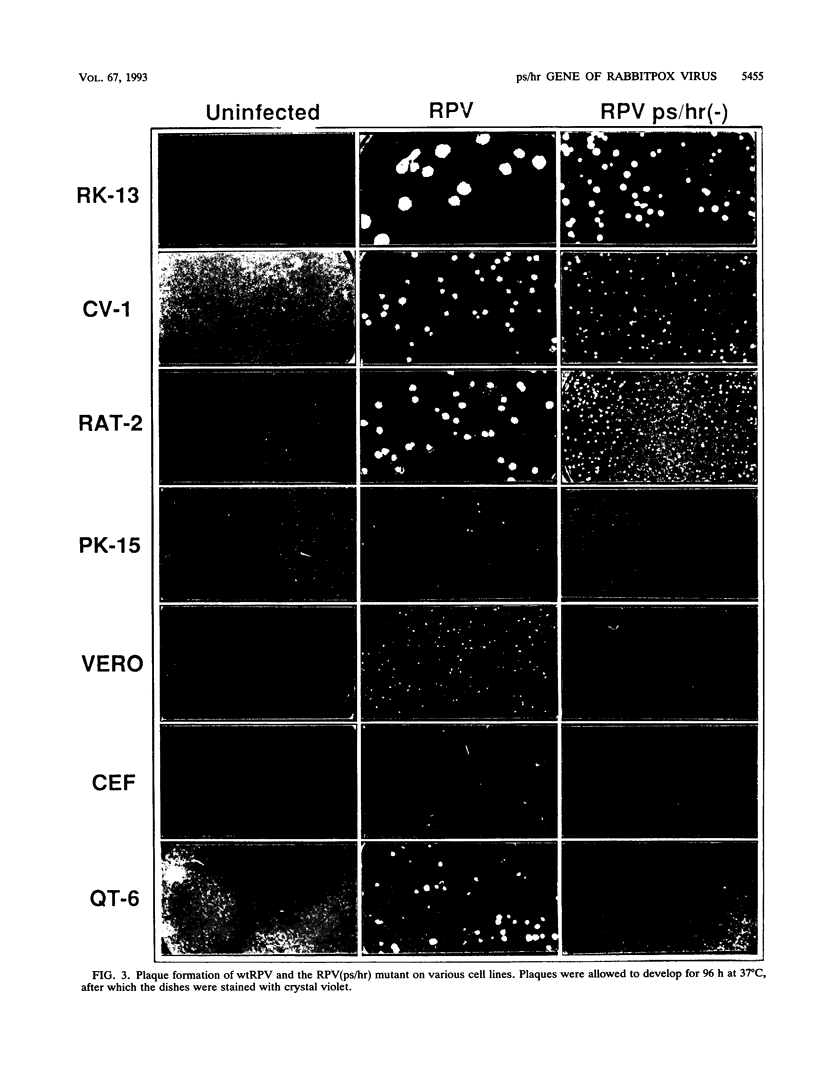
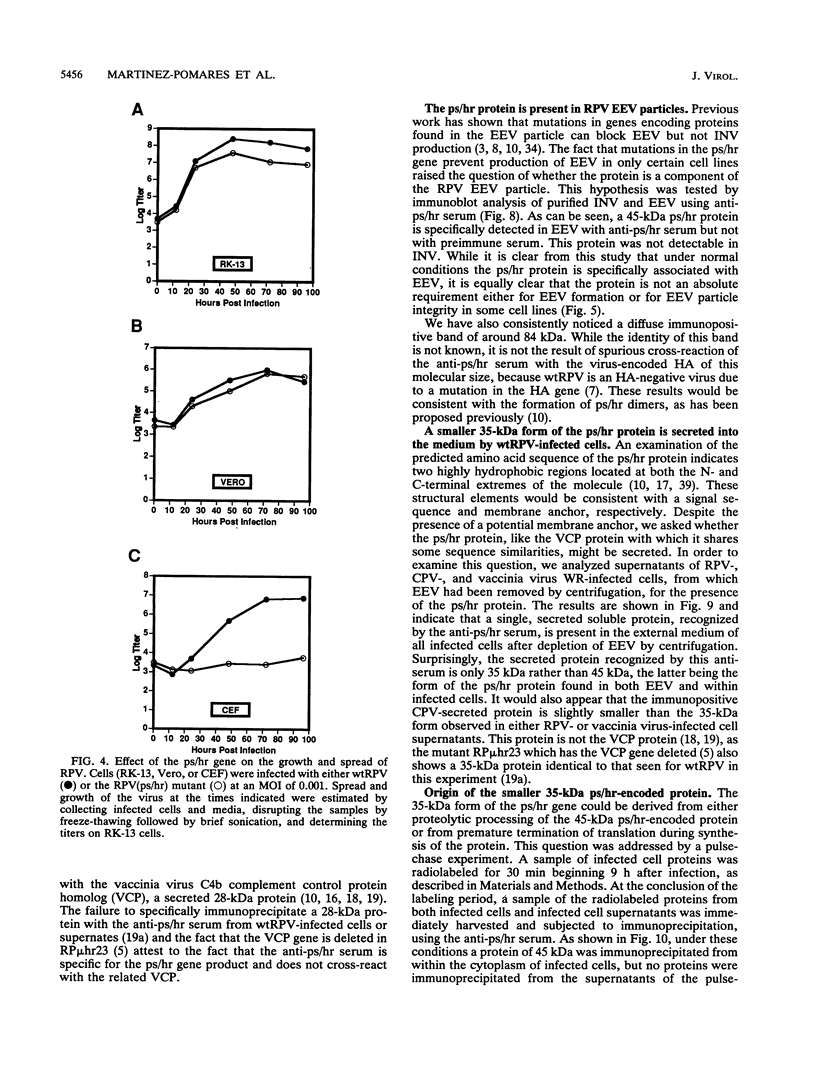
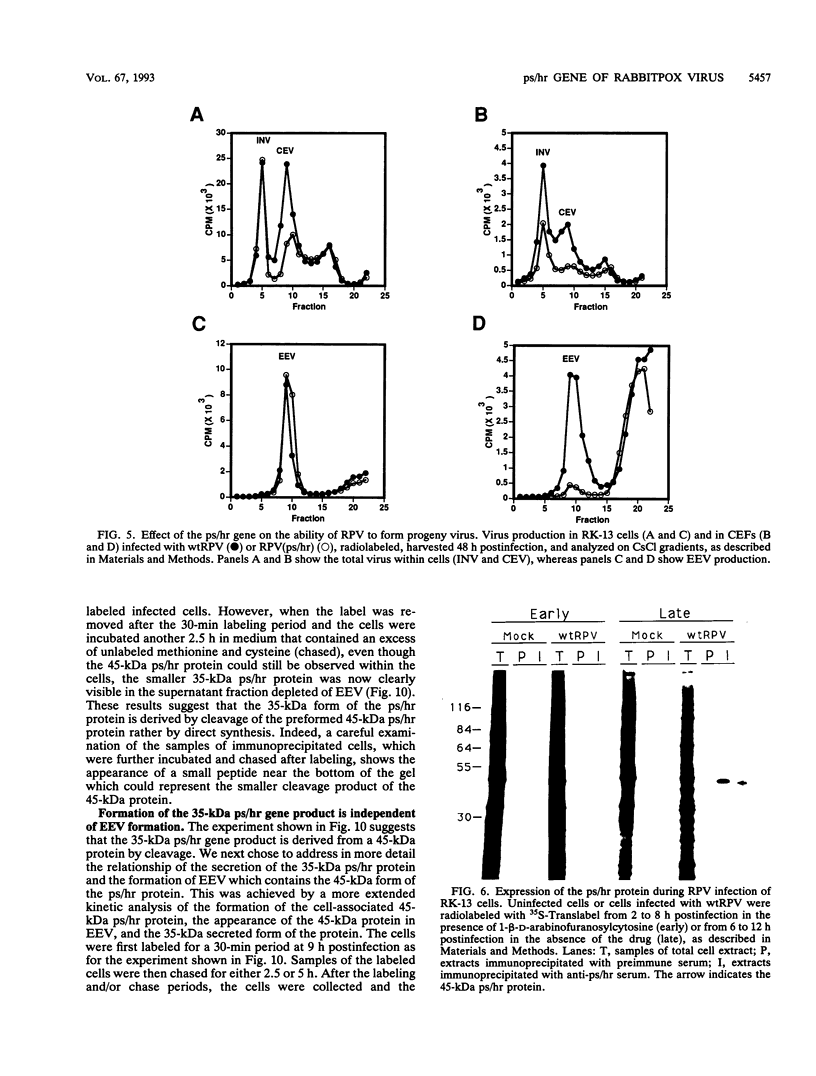
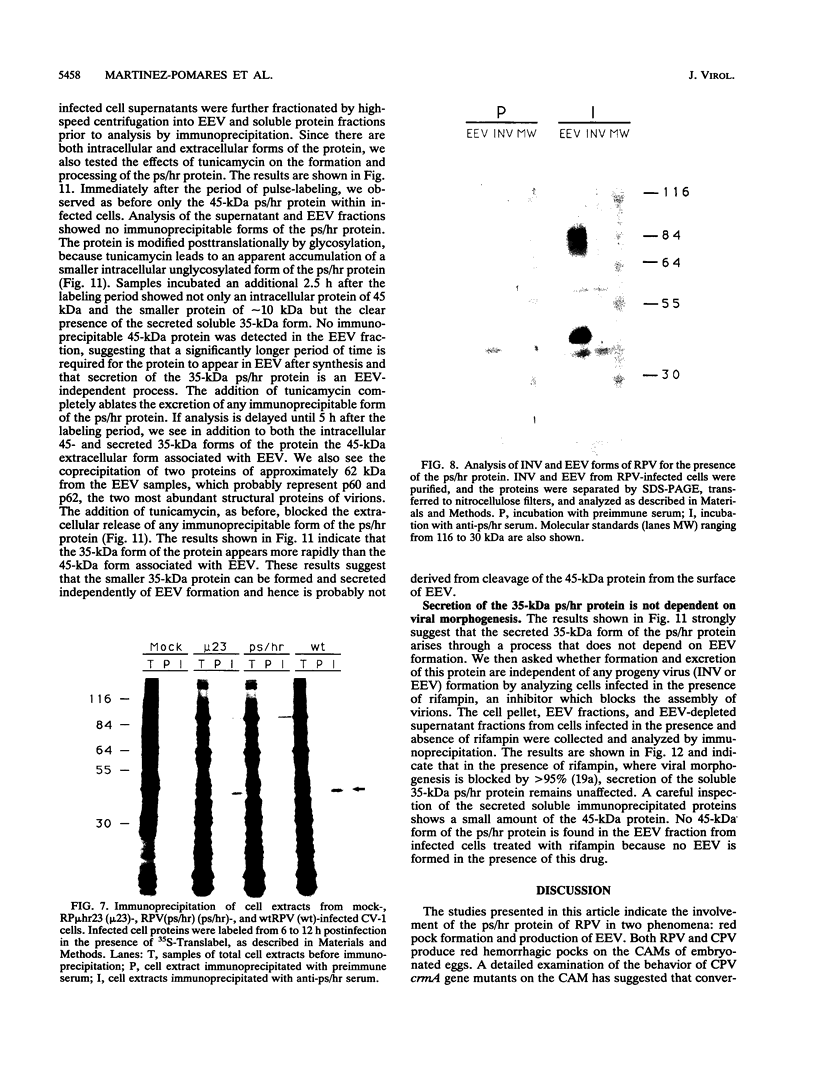
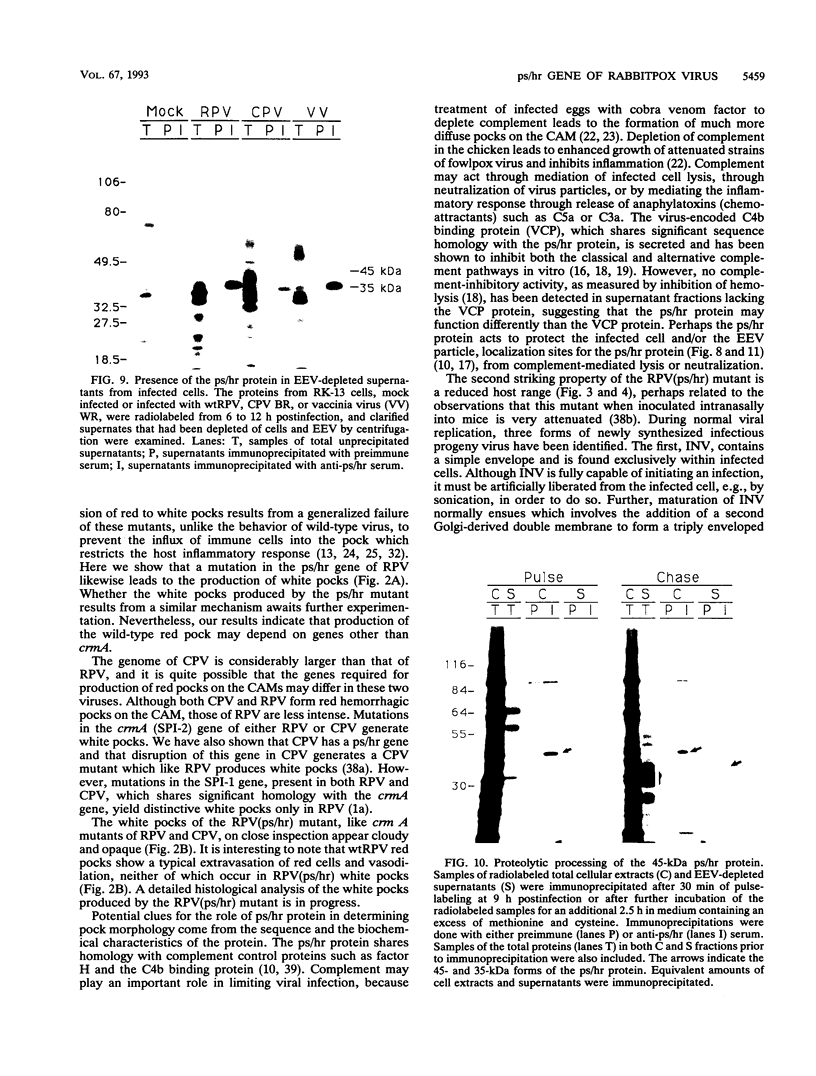
Wild-type rabbitpox virus (RPV) produces red hemorrhagic pocks on the chorioallantoic membranes (CAMs) of embryonated chicken eggs. Like the crmA (SPI-2) gene of cowpox virus, disruption of the RPV ps/hr gene results in a mutant which produces white pocks on the CAMs. An examination of the properties of the RPV(ps/hr) mutant in cell culture also reveals a significantly reduced host range, defined as the inability to form plaques, compared with wild-type virus. One of several cell types on which RPV(ps/hr) mutants fail to produce plaques is chicken embryo fibroblasts, cells which have been traditionally used to propagate spontaneously arising white pock mutants isolated from CAMs. The inability of the RPV(ps/hr) mutant to form plaques in chicken embryo fibroblasts correlates with a failure of a low multiplicity of infection to spread to neighboring cells and to form extracellular enveloped virus (EEV), although the formation and yields of infectious intracellular naked virus appear relatively normal. The gene product of the ps/hr gene, initially synthesized as a 45-kDa glycoprotein, is found as a component of EEV, but not intracellular naked virus, and as a smaller, secreted soluble protein of 35 kDa. Production of the secreted 35-kDa protein was found to be independent of any viral morphogenesis, suggesting two distinct pathways for release of the ps/hr gene product from the cell, i.e., as a component of the EEV particle and as a separately secreted glycoprotein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Blasco R., Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991 Nov;65(11):5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R., Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992 Jul;66(7):4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
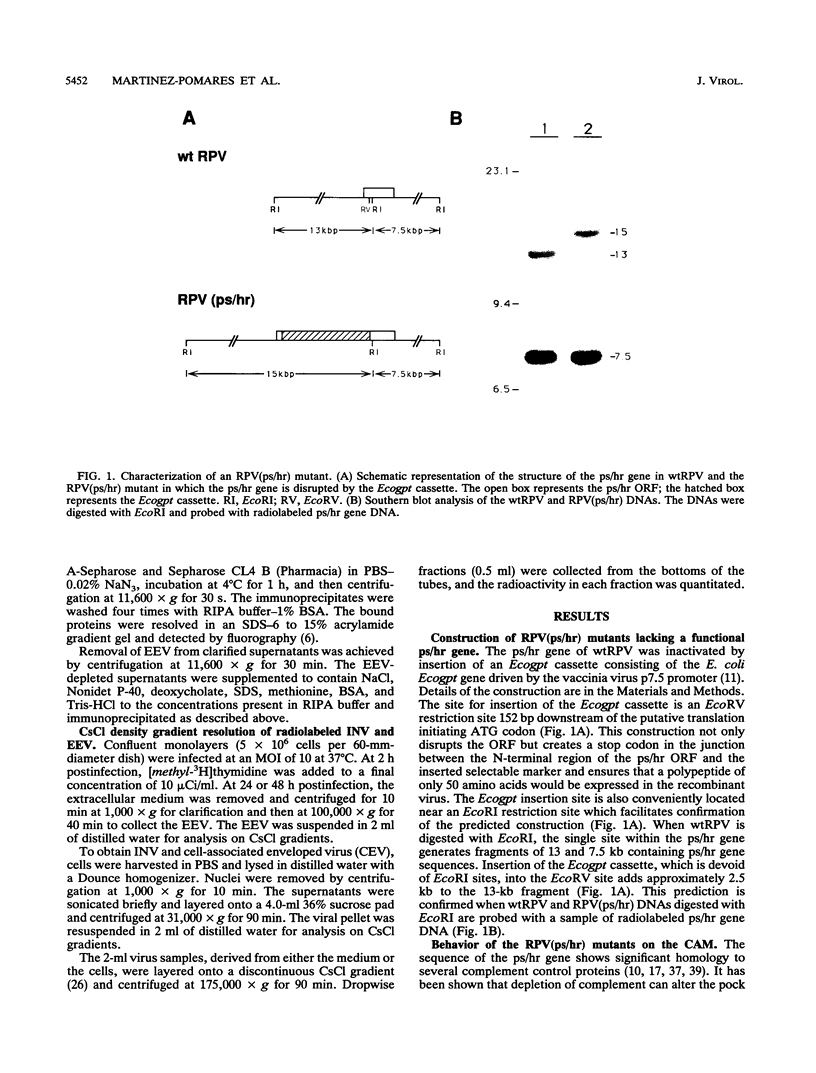
- Bloom D. C., Edwards K. M., Hager C., Moyer R. W. Identification and characterization of two nonessential regions of the rabbitpox virus genome involved in virulence. J Virol. 1991 Mar;65(3):1530–1542. doi: 10.1128/jvi.65.3.1530-1542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown C. K., Bloom D. C., Moyer R. W. The nature of naturally occurring mutations in the hemagglutinin gene of vaccinia virus and the sequence of immediately adjacent genes. Virus Genes. 1991 Jul;5(3):235–242. doi: 10.1007/BF00568973. [DOI] [PubMed] [Google Scholar]
- Duncan S. A., Smith G. L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992 Mar;66(3):1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. M., Andrews T. C., Van Savage J., Palmer P. S., Moyer R. W. Poxvirus deletion mutants: virulence and immunogenicity. Microb Pathog. 1988 May;4(5):325–333. doi: 10.1016/0882-4010(88)90060-5. [DOI] [PubMed] [Google Scholar]
- Engelstad M., Howard S. T., Smith G. L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992 Jun;188(2):801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- Engelstad M., Smith G. L. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993 Jun;194(2):627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Sambrook J. F. Conditional lethal mutants of rabbitpox virus. II. Mutants (p) that fail to multiply in PK-2a cells. Virology. 1966 Apr;28(4):600–609. doi: 10.1016/0042-6822(66)90245-5. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., Sechler J. M., Palumbo G. J., Albert J., Khairallah L. H., Buller R. M. Acute inflammatory response to cowpox virus infection of the chorioallantoic membrane of the chick embryo. Virology. 1992 Apr;187(2):693–704. doi: 10.1016/0042-6822(92)90472-2. [DOI] [PubMed] [Google Scholar]
- GEMMELL A., FENNER F. Genetic studies with mammalian poxviruses. III. White (u) mutants of rabbitpox virus. Virology. 1960 May;11:219–235. doi: 10.1016/0042-6822(60)90063-5. [DOI] [PubMed] [Google Scholar]
- Howard S. T., Chan Y. S., Smith G. L. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991 Feb;180(2):633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. N., Wolffe E. J., Payne L. G., Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992 Dec;66(12):7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Brown G. D., Graves R. L. The white pock mutants of rabbit poxvirus. II. The early white pock (mu) host range (hr) mutants of rabbit poxvirus uncouple transcription and translation in nonpermissive cells. Virology. 1980 Oct 30;106(2):234–249. doi: 10.1016/0042-6822(80)90247-0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Rothe C. T. The white pock mutants of rabbit poxvirus. I. Spontaneous host range mutants contain deletions. Virology. 1980 Apr 15;102(1):119–132. doi: 10.1016/0042-6822(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Ohta H., Yoshikawa Y., Kai C., Yamanouchi K., Taniguchi H., Komine K., Ishijima Y., Okada H. Effect of complement depletion by cobra venom factor on fowlpox virus infection in chickens and chicken embryos. J Virol. 1986 Feb;57(2):670–673. doi: 10.1128/jvi.57.2.670-673.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Yoshikawa Y., Yamanouchi K., Ishijima Y., Komine K., Okada H. Role of complement in the pathogenicity of fowlpox virus infection in chickens and chicken embryos. Nihon Juigaku Zasshi. 1988 Feb;50(1):145–152. doi: 10.1292/jvms1939.50.145. [DOI] [PubMed] [Google Scholar]
- Palumbo G. J., Buller R. M. Inhibitors of the lipoxygenase pathway specifically block orthopoxvirus replication. Virology. 1991 Jan;180(1):457–463. doi: 10.1016/0042-6822(91)90058-j. [DOI] [PubMed] [Google Scholar]
- Palumbo G. J., Pickup D. J., Fredrickson T. N., McIntyre L. J., Buller R. M. Inhibition of an inflammatory response is mediated by a 38-kDa protein of cowpox virus. Virology. 1989 Sep;172(1):262–273. doi: 10.1016/0042-6822(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkus M. E., Goebel S. J., Davis S. W., Johnson G. P., Limbach K., Norton E. K., Paoletti E. Vaccinia virus host range genes. Virology. 1990 Nov;179(1):276–286. doi: 10.1016/0042-6822(90)90296-4. [DOI] [PubMed] [Google Scholar]
- Perkus M. E., Goebel S. J., Davis S. W., Johnson G. P., Norton E. K., Paoletti E. Deletion of 55 open reading frames from the termini of vaccinia virus. Virology. 1991 Jan;180(1):406–410. doi: 10.1016/0042-6822(91)90047-f. [DOI] [PubMed] [Google Scholar]
- Pickup D. J., Ink B. S., Hu W., Ray C. A., Joklik W. K. Hemorrhage in lesions caused by cowpox virus is induced by a viral protein that is related to plasma protein inhibitors of serine proteases. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7698–7702. doi: 10.1073/pnas.83.20.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F., McClain M. E., Easterbrook K. B., McAuslan B. R. A mutant of its multiplication in three cell types. Virology. 1965 Aug;26(4):738–745. [PubMed] [Google Scholar]
- Schmutz C., Payne L. G., Gubser J., Wittek R. A mutation in the gene encoding the vaccinia virus 37,000-M(r) protein confers resistance to an inhibitor of virus envelopment and release. J Virol. 1991 Jul;65(7):3435–3442. doi: 10.1128/jvi.65.7.3435-3442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Wignall J. M., Din W. S., Farrah T., Upton C., McFadden G., Goodwin R. G. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991 Apr 15;176(1):335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Howard S. T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991 Jun;72(Pt 6):1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S. Two vaccinia virus proteins structurally related to the interleukin-1 receptor and the immunoglobulin superfamily. J Gen Virol. 1991 Mar;72(Pt 3):511–518. doi: 10.1099/0022-1317-72-3-511. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Hruby D. E., Maliszewski C. R., Pickup D. J., Sims J. E., Buller R. M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992 Oct 2;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Takahashi-Nishimaki F., Funahashi S., Miki K., Hashizume S., Sugimoto M. Regulation of plaque size and host range by a vaccinia virus gene related to complement system proteins. Virology. 1991 Mar;181(1):158–164. doi: 10.1016/0042-6822(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J Virol. 1992 Apr;66(4):2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. The molecular pathogenesis of poxviruses. Curr Top Microbiol Immunol. 1990;163:125–151. doi: 10.1007/978-3-642-75605-4_5. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Card J. P., Meade R. P., Robbins A. K., Enquist L. W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991 Mar;65(3):1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Tufaro F., Enquist L. W. A cellular function is required for pseudorabies virus envelope glycoprotein processing and virus egress. J Virol. 1992 Jun;66(6):3803–3810. doi: 10.1128/jvi.66.6.3803-3810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]