Abstract
The enzymes cyclooxygenase-1 and cyclooxygenase-2 (COX-1 and COX-2) catalyze the conversion of arachidonic acid to prostaglandin (PG) H2, the precursor of PGs and thromboxane. These lipid mediators play important roles in inflammation and pain and in normal physiological functions. While there are abundant data indicating that the inducible isoform, COX-2, is important in inflammation and pain, the constitutively expressed isoform, COX-1, has also been suggested to play a role in inflammatory processes. To address the latter question pharmacologically, we used a highly selective COX-1 inhibitor, SC-560 (COX-1 IC50 = 0.009 μM; COX-2 IC50 = 6.3 μM). SC-560 inhibited COX-1-derived platelet thromboxane B2, gastric PGE2, and dermal PGE2 production, indicating that it was orally active, but did not inhibit COX-2-derived PGs in the lipopolysaccharide-induced rat air pouch. Therapeutic or prophylactic administration of SC-560 in the rat carrageenan footpad model did not affect acute inflammation or hyperalgesia at doses that markedly inhibited in vivo COX-1 activity. By contrast, celecoxib, a selective COX-2 inhibitor, was anti-inflammatory and analgesic in this model. Paradoxically, both SC-560 and celecoxib reduced paw PGs to equivalent levels. Increased levels of PGs were found in the cerebrospinal fluid after carrageenan injection and were markedly reduced by celecoxib, but were not affected by SC-560. These results suggest that, in addition to the role of peripherally produced PGs, there is a critical, centrally mediated neurological component to inflammatory pain that is mediated at least in part by COX-2.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to treat inflammation and pain (1). The cardinal signs of inflammation, including edema, hyperalgesia, and erythema, develop as an acute response to a local inflammatory insult. These symptoms result from the action of inflammatory agents such as bradykinin, histamine, neurokinins, complement, and nitric oxide, which can originate locally or from cells that infiltrate the site of insult (2, 3). Elevated levels of prostaglandins (PGs) are also produced during inflammation (4) and enhance or prolong signals produced by pro-inflammatory agents, but alone do not cause inflammation (5–10). NSAIDs reduce or prevent the production of PGs by direct inhibition of the cyclooxygenase (COX) enzymes (11, 12). The observation that NSAIDs inhibit COX activity attests to the contribution of PGs to inflammation.
The COX enzymes catalyze the bis-oxygenation of free arachidonic acid to PGH2, the committed step in PG formation. PGH2 is converted into the other PGs or thromboxane (Tx)A2 by specific synthases (13). There are two COX enzymes, referred to as COX-1 and COX-2, which catalyze identical reactions (14–16). COX-1 is thought to produce PGs important for homeostasis and certain physiological functions and is expressed constitutively in most tissues and cells (17), although it can be induced in some cell lines under certain conditions (18). A second, inducible, form of COX was hypothesized to exist on the basis of the finding of a glucocorticoid-regulated increase in COX activity observed in vitro and in vivo in response to inflammatory stimuli (19, 20). The isolation of a distinct gene and enzyme for COX-2 confirmed this hypothesis and led to the supposition that selective inhibition of inducible COX-2 would be anti-inflammatory, while preserving the physiological functions of COX-1 derived PGs. This hypothesis was corroborated by the discovery and synthesis of anti-inflammatory compounds that selectively and potently inhibit COX-2 but not COX-1 (21–28). In contrast, NSAIDs inhibit both forms of COX at approximately equivalent concentrations (21, 29). Selective inhibition of COX-2 only partially reduces the level of PGs at sites of either acute or chronic inflammation, in comparison to NSAIDs, which reduce PGs to undetectable levels (23, 27, 30). Therefore, COX-1 may contribute significantly to the total pool of PG at a site of inflammation. To date, the contribution of COX-1 to inflammation has not been clearly established.
One approach to understanding the role of COX isoforms in inflammation is to use gene disruption technology in mice (knockouts). Using this approach, Langenbach et al. (31) reported that swelling elicited by application of arachidonic acid to the ear was diminished in COX-1-deficient mice compared with wild-type mice, suggesting a potential role for COX-1 in some forms of acute inflammation. Whether the results of this acute application of substrate represent events that occur in response to inflammatory stimuli is uncertain. To better understand the role of COX-1 in both inflammation and normal physiology, the anti-inflammatory and analgesic activities of SC-560 (COX-1 IC50 = 0.009 μM; COX-2 IC50 = 6.3 μM), a selective and potent inhibitor of COX-1, have been characterized. SC-560 provides a pharmacological tool to analyze the role of COX-1-derived PGs in inflammation and pain. The experiments reported herein using well established models of inflammation indicate that COX-1 may not play a significant role in acute inflammatory pain.
MATERIALS AND METHODS
In Vitro COX Enzyme Assay.
SC-560 was evaluated for COX inhibitory activity in vitro by using detergent-solubilized recombinant COX-1 and COX-2, as previously described (21). SC-560 [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole] or celecoxib (4-[5-(4-methylphenyl)-3-trifluoromethyl)-1H-pyrazol-1-yl]-benzenesulfonamide) (synthesized at Searle) at concentrations from 0.1 nM to 100 μM were pre-incubated with enzyme for 10 min before addition of arachidonic acid. PGE2 formed during a 10-min incubation with substrate was determined by ELISA (Cayman Chemicals, Ann Arbor, MI).
TxB2 Production in Whole Blood.
Whole blood from rats dosed for 4 hr with various amounts of indomethacin, SC-560, celecoxib, or vehicle (aqueous solution of 0.5% methocel, 0.025% Tween-20) was collected into heparinized tubes. Calcium ionophore A23187 (20 μg/ml final concentration, Sigma) was used to stimulate TxA2 production by incubation with blood for 10 min at 37°C, after which the samples were centrifuged (1,000 × g, 10 min, 4°C). Cellular protein in the supernatant was precipitated with methanol (1:5, supernatant:methanol, vol/vol) at room temperature for 10 min and removed by centrifugation (1000 × g, 10 min, 4°C). TxB2, the breakdown product of TxA2, was quantified in the final supernatant by ELISA (Cayman Chemicals, Ann Arbor, MI).
Reversed Passive Dermal Arthus Reaction.
The reversed dermal Arthus reaction in rats was conducted as previously described (32). Briefly, animals were fasted for 18 hr before compound administration; 1 hr after oral administration of compounds the animals were shaved mid-dorsally and placed in a restraint. The Arthus reaction was induced by intradermal injection of rabbit anti-chicken egg albumin IgG (0.8 mg/2 ml per site, Cappel) followed immediately by intravenous injection of 10 mg of chicken egg albumin (Sigma) in 0.5 ml of saline. Rats were euthanized by CO2 asphyxiation and the injury area was excised and placed on dry ice. Tissues were homogenized (Polytron LS10–35, Brinkmann) in 2 ml of methanol and adjusted to 16.7% methanol by the addition of water. The homogenate was cooled on ice for 30 min and centrifuged (2000 × g, 25 min, 4°C) to remove denatured protein. Supernatants were applied to a reverse-phase C18 Sep-Pak cartridge (Millipore) that was preconditioned with methanol followed by a water wash. The cartridges were washed sequentially with water, 10% methanol, and petroleum ether. Eicosanoids were eluted with methyl formate. The methyl formate extracts were evaporated under a stream of nitrogen and reconstituted with ELISA buffer. TxB2 and PGE2 were quantified by ELISA.
Air-Pouch Model of Inflammation.
An air cavity was produced by subcutaneous injection of 20 ml of sterile air into the intrascapular area of the back of male Lewis rats (175–200 g) (33). Animals were fasted 18 hr before compound administration. One day after initial air injection, compounds were administered orally and 1 hr later lipopolysaccharide (LPS, Escherichia coli strain 0111:β4) (2 ml of 1 μg/ml in saline) was injected into the pouch to produce an inflammatory reaction. After 3 hr of LPS treatment, 2 ml of 100 μM arachidonic acid was injected into the pouch, 15 min later animals were euthanized by CO2 asphyxiation, and the pouch fluid was collected for PG determination by ELISA. The stomachs of these animals were excised, opened, and cleaned, and the mucosal lining was dissected and frozen in liquid nitrogen. Stomach tissue was processed by homogenization in 70% ethanol and centrifuged, and the supernatants were dried under a stream of nitrogen. The samples were resuspended in ELISA buffer and PGs were quantified by ELISA.
Measurement of Edema, Hyperalgesia, and PG Production in the Carrageenan-Injected Rat Paw.
Paw edema was induced by injecting 0.1 ml of 1% carrageenan in saline into the hind paw of a male Sprague–Dawley rat (175–200 g) (34). Rats were fasted 18 hr before oral administration of compounds, and carrageenan was administered 1 hr later. Edema was measured with a water displacement plethysmometer (Ugo Basile, Varese, Italy) as change in paw volume. Hyperalgesia was quantified as a measure of the nociceptive response to a thermal stimulus from a radiant heat source (a high-intensity projector lamp bulb) positioned under a Plexiglas floor directly beneath the hind paw. The withdrawal latency of the carrageenan-injected paw was measured to the nearest 0.1 sec and compared with the response of the contralateral noninjected paw. At selected times after challenge, rats were asphyxiated with CO2 and their hind paws were removed and injected with 0.1 ml of 10 μM indomethacin in saline to prevent further production of eicosanoids. The paws were lacerated with a scalpel, suspended off the bottom of a polypropylene centrifuge tube with an Eppendorf pipette tip and centrifuged (1,800 × g, 15 min, 4°C) to express the inflammatory fluid. The volume expressed from each paw was determined and the fluid was analyzed by ELISA for PG production. In these same experiments, cerebrospinal fluid (CSF) was collected by making a skin incision on the dorsal midline of the head and exposing the cisternal membrane under the muscle at the base of the skull. A 27-gauge needle attached to a 1-ml syringe was used to puncture the cisternal membrane and collect the CSF. The spinal fluid was analyzed for PGs by ELISA.
All animal use described here was approved by the Monsanto Animal Care and Use Committee.
RESULTS
Inhibition of COX Activity in Vitro by a Selective COX-1 Inhibitor, SC-560.
The effect of SC-560 on recombinant human COX-1 and human COX-2 enzymatic activity was assessed in vitro and compared with the selective COX-2 inhibitor celecoxib (SC-58635) (22). Preincubation of COX-1 with SC-560 inhibited the conversion of arachidonic acid to PGE2 in a concentration-dependent manner. Half-maximal inhibition (IC50) of COX-1 was achieved with 0.009 μM SC-560, and more than 95% of COX-1 activity was inhibited with 0.3 μM. In contrast, more than 40% of the COX-2 activity remained at concentrations up to 100 μM SC-560. The IC50 of SC-560 for COX-2 was 6.3 μM, nearly 1,000-fold higher than with COX-1. The IC50 for celecoxib on COX-1 is 15 μM and on COX-2 is 0.04 μM (22). The structures of SC-560 and celecoxib are shown in Fig. 1.
Figure 1.
Structures of SC-560 and celecoxib. SC-560: COX-1 IC50 = 0.009 μM, COX-2 IC50 = 6.3 μM; celecoxib: COX-1 IC50 = 15 μM, COX-2 IC50 = 0.04 μM.
Inhibition of COX Activity in an ex Vivo-Stimulated Whole Blood Assay.
The oral bioavailability and intrinsic potency of SC-560 were evaluated by using a whole blood assay of platelet COX activity. TxB2 production from whole blood platelets stimulated with calcium ionophore (A23187) was used as a measure of COX-1 activity, since platelets do not express COX-2. A23187-stimulation of rat whole blood resulted in a 6-fold increase in TxB2 production compared with control treated blood (Table 1). Oral dosing with either 10 or 30 mg/kg SC-560 1 hr before assay completely inhibited ionophore-stimulated TxB2 production (Table 1), indicating that SC-560 was orally bioavailable and inhibited COX-1 in vivo. A 10-mg/kg dose of SC-560 was as effective in this assay as a 5-mg/kg dose of indomethacin, a potent mixed inhibitor of COX-1 and COX-2. Oral administration of either 10 or 30 mg/kg of the selective COX-2 inhibitor celecoxib had no effect on stimulated whole blood TxB2 production. The celecoxib plasma concentration after a 10-mg/kg oral dose was 6 μg/ml by HPLC analysis (data not shown), confirming its oral bioavailability and exposure in blood.
Table 1.
Inhibition of TxB2 in ex vivo calcium ionophore-stimulated whole blood
| TxB2 ng/ml
|
||
|---|---|---|
| −Ionophore | +Ionophore | |
| Vehicle | 0.65 ± 0.1 | 4.5 ± 0.8 |
| Indomethacin (10 mg/kg) | 0.92 ± 0.2 | |
| SC-560 (30 mg/kg) | 0.62 ± 0.1 | |
| Celecoxib (30 mg/kg) | 4.4 ± 1.4 | |
Compounds were administered orally 1 hr before sample collection. Whole blood collected from animals (n = 4) was stimulated with calcium ionophore A23187. Plasma was prepared and TxB2 was quantified by ELISA. Data are reported as the mean ± SEM.
Reversed Passive Dermal Arthus Reaction.
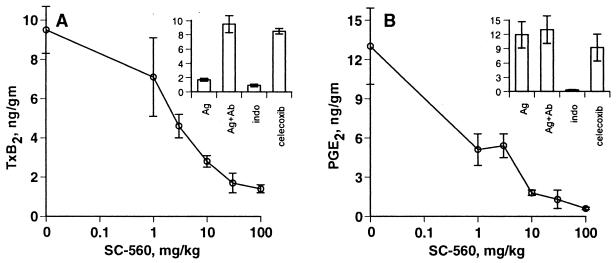
Dermal vascular injury was induced in the rat by the intradermal injection of rabbit anti-egg albumin IgG, followed by intravenous injection of egg albumin. In this model, tissue TxB2 levels are elevated 2 hr after antigen injection, plateau between 3 and 5 hr, and remain elevated beyond 7 hr (35). In the experiments reported here, a 10- to 15-fold increase in tissue TxB2 above normal levels was obtained 4 hr after antigen injection (Fig. 2A Inset). Oral administration of SC-560 blocked TxB2 production in a dose-dependent manner in the inflamed skin (Fig. 2A), with maximum inhibition obtained with both SC-560 and indomethacin. The reversed dermal Arthus reaction did not cause an increase in PGE2 above basal levels (Fig. 2B Inset). Nevertheless, basal PGE2 was inhibited by SC-560 at doses similar to those that caused TxB2 inhibition (Fig. 2B). Celecoxib had no effect on TxB2 production or basal levels PGE2 in this model (Fig. 2 A and B, Insets). These data suggest that in the dermal Arthus reaction, TxB2 and PGE2 are produced by COX-1. Neither SC-560 nor celecoxib inhibited leukotriene B4 production in this model (data not shown).
Figure 2.
Inhibition of PG production in the reversed passive dermal Arthus reaction. Compounds were administered by oral gavage 1 hr before initiation of the Arthus reaction. The Arthus reaction was initiated by intradermal injection of rabbit anti-chicken egg albumin (Ab) followed immediately by i.v. injection of 10 mg of chicken egg albumin (Ag). Dermal injection sites were removed and extracted for PGs, which were quantified by ELISA. (A) Sensitivity of Arthus reaction-stimulated TxB2 to SC-560. (Inset) TxB2 levels in negative control (Ag only) and Arthus reaction skin (Ag + Ab) compared with Arthus reaction skin from animals dosed with 10 mg/kg indomethacin or 30 mg/kg celecoxib. (B) Sensitivity of rat skin PGE2 to SC-560. (Inset) PGE2 levels in control skin and Arthus reaction skin compared with Arthus reaction skin from animals dosed with 10 mg/kg indomethacin or 30 mg/kg celecoxib. Data are expressed as mean ± SEM, n = 4 per treatment group.
Rat Air Pouch Model of LPS-Induced Inflammation.
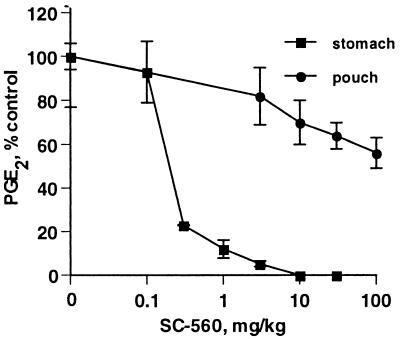
The effect of SC-560 on inflammatory PG production in the LPS-induced rat air pouch was assessed; PG production in the inflamed air pouch model is driven by COX-2. Like the carrageenan air pouch model previously described (26), introduction of LPS into an established subcutaneous air pouch in rats induced marked COX-2 expression (data not shown). Addition of arachidonic acid to the LPS-treated air pouch after COX-2 induction resulted in substantial PG production. Administration of a high dose of SC-560 (100 mg/kg) before LPS had little effect on PGE2 production in the pouch (approximately 40% inhibition at 100 mg/kg, Fig. 3). In contrast, SC-560 potently inhibited PGE2 production in stomach mucosa (ED50 = 0.2 mg/kg), as expected because COX-1 is the primary source of gastric mucosa PGs. The results with SC-560 indicated that COX-2 is responsible for PG production in the LPS-induced rat air pouch (26).
Figure 3.
Inhibition of PG production in the inflamed rat air pouch and stomach mucosa by SC-560. SC-560 was administered by oral gavage 1 hr before initiating an inflammatory response in the air pouch with LPS. PGE2 present in the pouch exudate (•) and extracted from the stomach mucosa (■) was quantified by ELISA. Each point represents percent of control PGE2 ± SEM, n = 6 per treatment group.
Carrageenan-Induced Inflammation in the Rat Footpad.
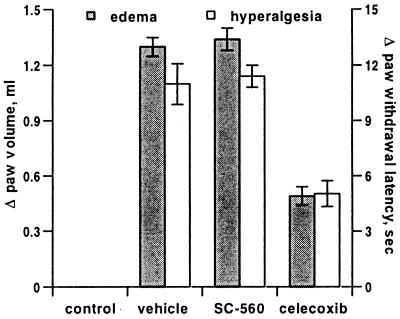
Results presented thus far establish SC-560 as a unique pharmacological tool that potently and selectively inhibited COX-1 produced PG in vivo without affecting COX-2-produced PG. SC-560 was then used to investigate the role of COX-1-derived PGs in the carrageenan-inflamed rat footpad (27, 30, 34). This model allowed evaluation of inflammatory pain and swelling secondary to PG production. A prophylactic dose of 30 mg/kg SC-560 had no significant effect on the onset of either hyperalgesia or edema (Fig. 4), whereas celecoxib prevented the full manifestation of these inflammatory symptoms. This result supports previous studies demonstrating that inflammation in this model is driven by COX-2 (27, 36).
Figure 4.
Effect of COX inhibitors on carrageenan-induced edema and hyperalgesia in the rat footpad. A single dose of 30 mg/kg SC-560, celecoxib, or vehicle was administered by oral gavage 2 hr before initiation of inflammation with carrageenan. Edema (■, left axis) and hyperalgesia (□, right axis) were measured 6 hr after carrageenan injection. Data shown are the mean (n = 5) ± SEM and are from one of three experiments with similar results.
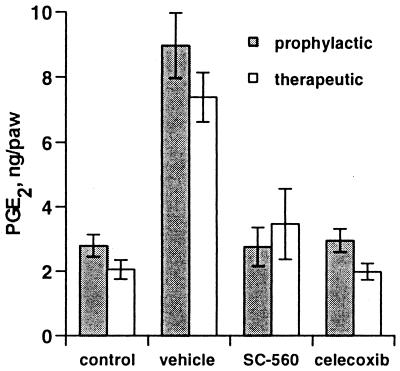
Carrageenan injection into the footpad causes a 4- to 5-fold increase in PGs. Previous data indicated that this increase was prevented by NSAIDs and by COX-2 inhibitors (27, 30, 34). NSAIDs reduce PGE2 level in the inflamed rat footpad to below baseline, whereas selective COX-2 inhibitors prevent the increase in PGE2 expression above basal levels (27, 36). The effect of selective COX-1 and COX-2 inhibitors on PGE2 production in the inflamed footpad was assessed. PGE2 level in the carrageenan-challenged footpads from animals dosed prophylactically with SC-560 was nearly the same as that in animals dosed with celecoxib (Fig. 5). This result suggests that either COX-1 or COX-2 inhibition can prevent carrageenan-induced increases in PGs above basal levels, yet only COX-2 inhibition prevented the development of edema and hyperalgesia. To further examine this phenomenon, the effect of therapeutic administration of SC-560 on reversing elevated PG levels in the inflamed rat footpad was evaluated. A therapeutic dose of SC-560 did not reverse either hyperalgesia or edema (not shown) but did decrease PGE2 in the inflamed footpad to a level comparable to that in a COX-2-inhibited animal (Fig. 5). Celecoxib was an effective analgesic when administered therapeutically, but was not able to reverse established edema during the time course of these experiments, consistent with previous results (36).
Figure 5.
Effect of COX inhibitors on carrageenan-induced PGE2 in the rat footpad. A single dose of 30 mg/kg SC-560, celecoxib, or vehicle was administered by oral gavage either 2 hr before (■) or 3 hr after (□) carrageenan injection. Paw inflammatory fluid was collected and PGE2 was quantified by ELISA. Data represent mean (n = 5) ± SEM and are from one of three experiments with similar results.
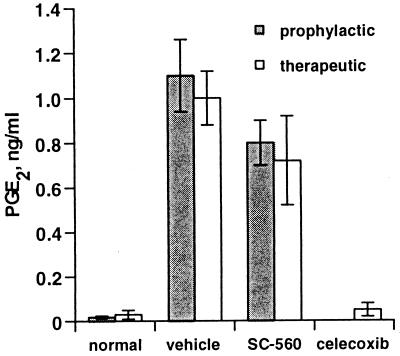
Because of the paradoxical effect of selective COX inhibitors on local PG production, the carrageenan footpad model of inflammation was used to examine PG production at another potential site of action. Work from Yaksh and coworkers has shown that NSAIDs can inhibit hyperalgesic responses when administered directly to the spinal cord (37, 38), and increases in COX-2 mRNA have been observed after peripheral stimulation (39, 40); these results suggest a role for central nervous system (CNS)-derived PGs in some pain responses. In preliminary studies, we found that injection of carrageenan into the footpad caused marked increases in PGE2 levels in CSF. Therefore, the effect of SC-560 or celecoxib on carrageenan-stimulated CSF PGE2 levels was assessed. Administration of SC-560 either prophylactically or therapeutically had no effect on CSF PGE2 levels, whereas celecoxib reduced PGE2 levels to background (Fig. 6). SC-560 was detected in the spinal tissue at a concentration slightly higher than that detected in the plasma (270 ng/g of spinal cord tissue vs. 109 ng/ml of plasma at 30 mg/kg), confirming tissue penetration at a pharmacologically effective concentration.
Figure 6.
Effect of COX inhibitors on PGE2 in rat CSF. A single dose of 30 mg/kg SC-560, celecoxib, or vehicle was administered by oral gavage either 2 hr before (■) or 3 hr after (□) carrageenan injection. CSF was collected and PGE2 was quantified by ELISA. Data represent several experiments reported as the mean (n = 5–15) ± SEM.
DISCUSSION
The description of inflammation- and glucocorticoid-regulated COX activity suggested a therapeutic target separate from the COX activity responsible for maintaining normal physiology (19, 26). Cloning of the COX-2 gene coding for a second COX enzyme identified the target responsible for increased COX activity related to inflammation. NSAIDs were observed to inhibit both COX-1 and COX-2, suggesting that, along with the beneficial therapeutic effect of inhibiting COX-2, their detrimental side effects were a result of inhibiting COX-1 (21–27). Selective inhibition of COX-2 clearly demonstrated its role in inflammation (24, 27), but these studies did not address the potential role of COX-1 in inflammation. Recently, the relative biological role of COX-1 in inflammation was evaluated by using gene-targeted mice in which the gene expressing COX-1 was disrupted (31). It was reported that arachidonic acid-induced ear swelling in COX-1-deficient mice was reduced compared with wild-type mice, suggesting a role for COX-1 in this model (31). COX-1 is expressed constitutively in mouse skin and, because substrate was applied directly to the tissue, the increased PG production and inflammation in the normal mouse and lack thereof in the COX-1 knockout would be expected; moreover, the experimental time frame (2 hr) may not have been sufficient for COX-2 induction. Nevertheless, the results in the COX-1-deficient mice have raised questions about the role of this enzyme in inflammation. The present study was designed to investigate the role of COX-1-derived PGs in inflammation and pain with a pharmacological approach, by using a selective COX-1 inhibitor, SC-560. In in vitro studies, SC-560 was greater than 1,000-fold more potent as an inhibitor of COX-1 vs. COX-2. Demonstration of the oral bioavailability and in vivo activity of this selective and potent COX-1 inhibitor thus suggested that it could be an important pharmacological tool for analysis of the role of COX-1-derived PGs in several well characterized inflammation models.
Oral administration of SC-560 at 10 mg/kg produced maximal inhibition of platelet TxB2, equivalent to that observed with the potent NSAID indomethacin, which established the bioavailability of this compound. The lack of effect of celecoxib, a selective COX-2 inhibitor, independently confirms that COX-1 is responsible for TxB2 production in this assay. The intrinsic selectivity and potency of SC-560 in the in vitro enzyme assay and the ex vivo-stimulated whole blood assay suggested that it would be useful for evaluating the role of COX-1 in various inflammation models. To further characterize the in vivo activity of SC-560, two models of eicosanoid production were evaluated, one driven by COX-1 and the other by COX-2. The reversed dermal Arthus reaction is a skin inflammation model initiated by antigen/antibody interaction, which results in increased levels of tissue TxB2 and leukotriene B4 (35). SC-560, but not celecoxib, inhibited TxB2 production stimulated by antigen injection, suggesting that this eicosanoid is derived from COX-1 in the Arthus reaction. In other data not shown, the cellular source of TxB2 in the Arthus reaction was investigated. Dosing animals with aspirin 24 hr before initiating the Arthus reaction inhibited skin TxB2 to a lesser extent than that inhibited in serum (4% vs. 42% at 10 mg/kg and 58% vs. 88% at 100 mg/kg), while having little to no effect on skin PGE2. This result suggests that part but not all of the Arthus-reaction skin TxB2 is derived from platelets. Further evidence for the prominent role of COX-1 in production of dermal PGs came from analysis of PGE2 levels, which were found at high basal levels in normal rat skin but were not increased during the Arthus reaction. Basal PGE2 levels in skin were markedly reduced by SC-560 administration but were not affected by celecoxib, suggesting that PGE2 derives from COX-1 rather than COX-2 in this tissue. In addition to increased TxB2 production, the Arthus reaction triggers a cascade of inflammatory responses that lead to vascular injury, including binding of the antigen/antibody complex to the Fc receptor, activation of complement, neutrophil adhesion and invasion, and increased expression of the chemotactic factor leukotriene B4. Neither COX inhibitor had any effect on leukotriene B4 levels, demonstrating that these compounds do not inhibit 5-lipoxygenase at maximally efficacious doses.
The effect of SC-560 and celecoxib on gastric PG production was also assessed as a measure of activity on COX-1, since COX-1 expression predominates in this tissue (26). In the present study, a dose of only 0.3 mg/kg SC-560 inhibited nearly 80% of the formation of PGE2 extracted from the stomach mucosa. The sensitivity of stomach PGs to the COX-1 inhibitor SC-560, but not to celecoxib, further supports the hypothesis that COX-1 mediates mucosal PG production.
To establish whether SC-560 was selective for COX-1 in vivo, its effect on PG production in the LPS-stimulated rat air pouch model was evaluated. The air pouch lining formed in the subcutaneous skin before LPS treatment is populated with macrophages and freshly dividing fibroblasts and is newly vascularized (26, 33). LPS treatment, unlike carrageenan treatment, did not cause cellular influx or fluid or eicosanoid accumulation in the pouch; however, LPS, like carrageenan, stimulated increased expression of COX-2 (Daniela Salvemini, personal communication). Addition of arachidonic acid to the LPS pouch resulted in marked PG production that was sensitive to selective COX-2 inhibitors. SC-560 was not effective in blocking PG production in the LPS pouch, whereas celecoxib is a potent inhibitor in this setting, establishing that SC-560 is a selective inhibitor of COX-1 in vivo.
The observation that SC-560 selectively inhibited COX-1 both in vitro and in vivo suggested that SC-560 would be a useful tool for assessing the role of COX-1 in standard models of inflammation and pain, such as carrageenan footpad edema, a model widely used for assessment of the anti-inflammatory and analgesic effects of NSAIDs (27, 34). Prophylactic administration of SC-560 had no effect on carrageenan-induced edema and hyperalgesia, whereas the selective COX-2 inhibitor celecoxib was fully active in this model. Prophylactic administration of dexamethasone or celecoxib produces similar effects on edema, hyperalgesia, and PG levels in this model (36). Previous results of Zhang et al. showed that therapeutic administration of celecoxib after inflammation was fully established reduced PG levels in the exudate from inflamed footpads to control values; the nonspecific COX inhibitor ketorolac reduced PG content to undetectable levels (36). Both ketorolac and celecoxib reduced hyperalgesia in this therapeutic model. Paradoxically, therapeutic administration of SC-560 reduced PGE2 levels in this experimental model to the same extent as was observed with celecoxib treatment, but did not affect established hyperalgesia. This result suggests that tissue levels of PGs at the site of inflammation may not fully reflect the biological response observed with systemic COX-2 inhibition. Nevertheless, it appears that COX-1-derived PGs do not mediate inflammation in the carrageenan rat footpad model. Previous results of Portanova et al. (30) demonstrated that neutralization of PGE2 with a specific monoclonal antibody prevented carrageenan-induced edema and hyperalgesia, and Zhang et al. (36) showed that the anti-PGE2 antibody reversed already established hyperalgesia. These results suggest that PGE2 produced in the periphery is essential to establish and maintain inflammatory hyperalgesia. Data presented herein, however, suggest that COX-2-derived PGs are distinguished from those synthesized by COX-1. It is possible that the PGs produced by COX-2 are compartmentalized in the footpad or that there is induction of COX-2 at another site that influences inflammation in the paw.
CNS PGs have been shown to be important in both thermal and mechanical hyperalgesia (41). Furthermore, COX-2 mRNA can be induced in the spinal cord after peripheral inflammation (39, 40, 42), and selective inhibitors of COX-2 applied directly to the lumbar spinal cord prevented inflammatory hyperalgesia (43). These observations point to a role for COX-2 expressed in the spinal cord in hyperalgesia. Our finding that peripheral inflammation causes marked increase in CSF PG levels that are exquisitely sensitive to COX-2 inhibition further supports a role for COX-2 in the CNS in pain transmission. Whether the PG we found in the CSF derives from the spinal cord or from higher centers is uncertain, since sampling was from the cisterna near the fourth ventricle. In any case, levels of PG in the CSF more closely reflected the biological response achieved with the isoform-selective COX inhibitors.
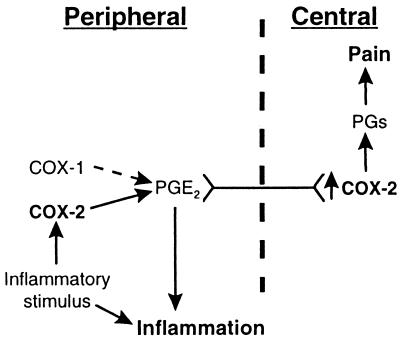
In summary, we have thoroughly evaluated the biochemical and anti-inflammatory activity of a selective COX-1 inhibitor in vivo. Data reported herein indicate that a selective COX-1 inhibitor attenuated PG and Tx production in a skin inflammation model characterized by vascular damage, plasma extravasation, and cellular influx, but had no substantial anti-inflammatory or analgesic effect in a model driven by COX-2. The observation that centrally derived PG levels provide a better marker than peripheral PGs for the analgesic effects of selective COX-2 inhibitors suggests that COX-2 activity in the CNS may play an important role in the induction and maintenance of the hyperalgesic state (Fig. 7).
Figure 7.
Model for COX-1- and COX-2-derived PGs in inflammation and pain. The initial peripheral inflammatory signal causes local upregulation of COX-2 and PGs that mediate plasma extravasation and neurological signals to the CNS. Inflammatory mediators including PGs increase nerve traffic, resulting in increased COX-2 expression in the spinal cord, which in turn produces PGs that influence central pain signals.
Acknowledgments
We thank Dr. Joseph Portanova for his excellent critique.
ABBREVIATIONS
- COX
cyclooxygenase
- PG
prostaglandin
- NSAID
nonsteroidal anti-inflammatory drug
- LPS
lipopolysaccharide
- CNS
central nervous system
- CSF
cerebrospinal fluid
References
- 1. Insel P A. In: The Pharmacological Basis of Therapeutics. Gilman A, Rall T W, Nies A S, Taylor P, editors. Oxford: Pergamon; 1990. pp. 638–681. [Google Scholar]
- 2.Garrison J C. In: The Pharmacological Basis of Therapeutics. Gilman A, Rall T W, Nies A S, Taylor P, editors. Oxford: Pergamon Press; 1990. pp. 574–599. [Google Scholar]
- 3.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 4.Davies P, MacIntyre D E. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 123–137. [Google Scholar]
- 5.Williams T J, Morley J. Nature (London) 1973;246:215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- 6.Williams T J. Br J Pharmacol. 1979;65:517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moncada S, Ferreira S H, Vane J R. Nature (London) 1973;246:217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira S H. Nat New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira S H, Nakamura M, de Abreu Castro M S. Prostaglandins. 1978;16:31–37. doi: 10.1016/0090-6980(78)90199-5. [DOI] [PubMed] [Google Scholar]
- 10.Davies P, Bailey P J, Goldenberg M M, Ford-Hutchinson A W. Annu Rev Immunol. 1984;2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 11.Smith J B, Willis A L. Nat New Biol. 1971;231:235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- 12.Vane J R. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 13.Smith W L, Marnett L J. Biochim Biophys Acta. 1991;1083:1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- 14.Xie W L, Chipman J G, Robertson D L, Erikson R L, Simmons D L. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Banion M K, Winn V D, Young D A. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kujubu D A, Fletcher B S, Varnum B C, Lim R W, Herschman H R. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 17.O’Neill G, Ford-Hutchinson A W. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 18.Smith C J, Morrow J D, Robert L J, II, Marnett L J. Biochem Biophys Res Commun. 1993;192:787–793. doi: 10.1006/bbrc.1993.1483. [DOI] [PubMed] [Google Scholar]
- 19.Fu J Y, Masferrer J L, Seibert K, Raz A, Needleman P. J Biol Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- 20.Masferrer J L, Seibert K, Zweifel B, Needleman P. Proc Natl Acad Sci USA. 1992;89:3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gierse J K, Hauser S D, Creely D P, Koboldt C, Rangwala S H, Isakson P C, Seibert K. Biochem J. 1995;305:479–484. doi: 10.1042/bj3050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penning T D, Talley J J, Bertenshaw S R, Carter J S, Collins P W, Docter S, Graneto M J, Lee L F, Malecha J W, Miyashiro J M, et al. J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 23.Anderson G D, Hauser S D, McGarity K L, Bremer M E, Isakson P C, Gregory S A. J Clin Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan C C, Gordon R, Brideau C, Rodger I W, Li C S, Prasit P. Can J Physiol Pharmacol. 1994;72:11–18. doi: 10.1139/y94-003. [DOI] [PubMed] [Google Scholar]
- 25.Li C S, Black W C, Chan C C, Ford-Hutchinson A W, Gauthier J Y, Gordon R, Guay D, Kargman S, Lau C K, Mancini J, et al. J Med Chem. 1995;38:4897–4905. doi: 10.1021/jm00025a007. [DOI] [PubMed] [Google Scholar]
- 26.Masferrer J L, Zweifel B S, Manning P T, Hauser S D, Leahy K M, Smith W G, Isakson P C, Seibert K. Proc Natl Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Proc Natl Acad Sci USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Futaki N, Takahashi S, Yokoyoma M, Arai I, Higuchi S, Otomo S. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 29.Meade E A, Smith W L, DeWitt D L. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 30.Portanova J P, Zhang Y, Anderson G D, Hauser S D, Masferrer J L, Seibert K, Gregory S A, Isakson P C. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langenbach R, Morham S G, Tiano H F, Loftin C D, Ghanayem B I, Chulada P C, Mahler J F, Lee C A, Goulding E H, Kluckman K D, et al. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan M S, Ward P A. J Immunol. 1992;149:331–339. [PubMed] [Google Scholar]
- 33.Sedgwick A D, Sin Y M, Edwards J C, Willoughby D A. J Pathol. 1983;141:483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 35.Smith W G, Shaffer A F, Currie J L, Thompson J M, Kim S, Rao T, Isakson P C. J Pharmacol Exp Ther. 1995;275:1332–1338. [PubMed] [Google Scholar]
- 36.Zhang Y, Shaffer A, Portanova J, Seibert K, Isakson P C. J Pharmacol Exp Ther. 1998;283:1069–1075. [PubMed] [Google Scholar]
- 37.Malmberg A B, Yaksh T L. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 38.Malmberg A B, Yaksh T L. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- 39.Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- 40.Ichitani Y, Shi T, Haeggstrom J Z, Samuelsson B, Hokfelt T. NeuroReport. 1997;8:2949–2952. doi: 10.1097/00001756-199709080-00028. [DOI] [PubMed] [Google Scholar]
- 41.Taiwo Y O, Levine J D. Brain Res. 1986;373:81–84. doi: 10.1016/0006-8993(86)90317-3. [DOI] [PubMed] [Google Scholar]
- 42.Yang L C, Marsala M, Yaksh T L. Pain. 1996;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- 43.Dirig D M, Konin G P, Isakson P C, Yaksh T L. Eur J Pharmacol. 1997;331:155–160. doi: 10.1016/s0014-2999(97)01053-4. [DOI] [PubMed] [Google Scholar]