Abstract
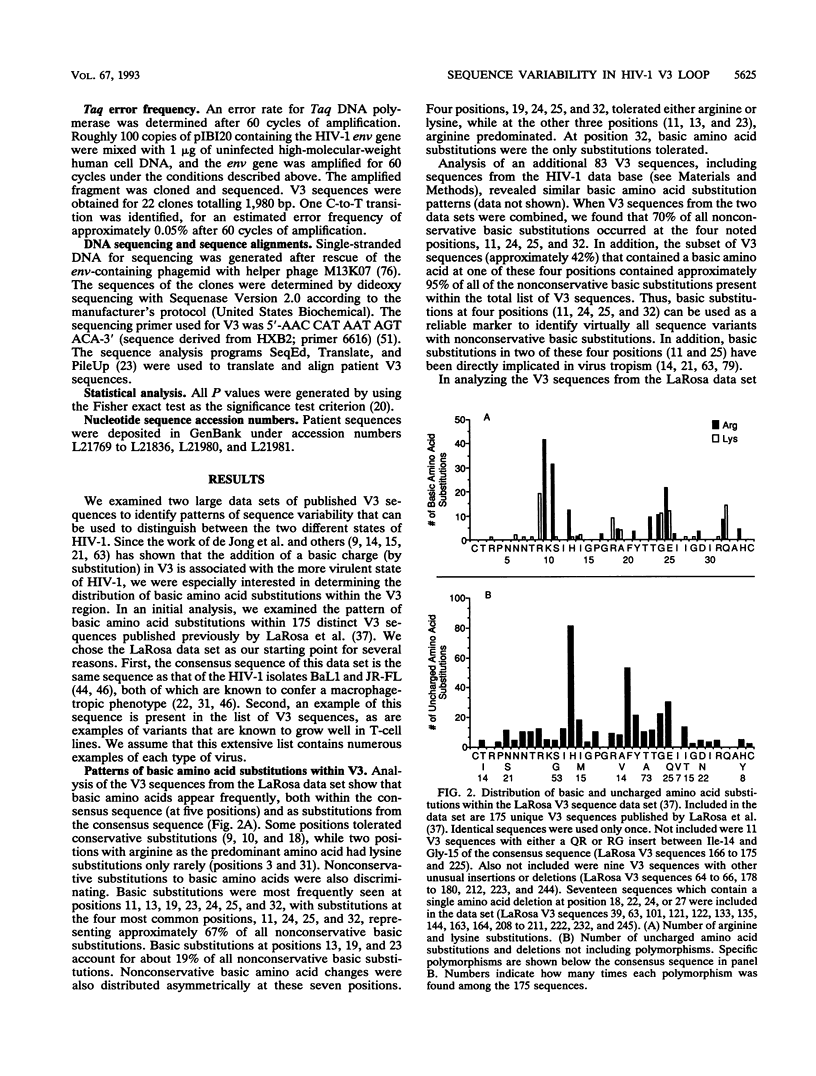
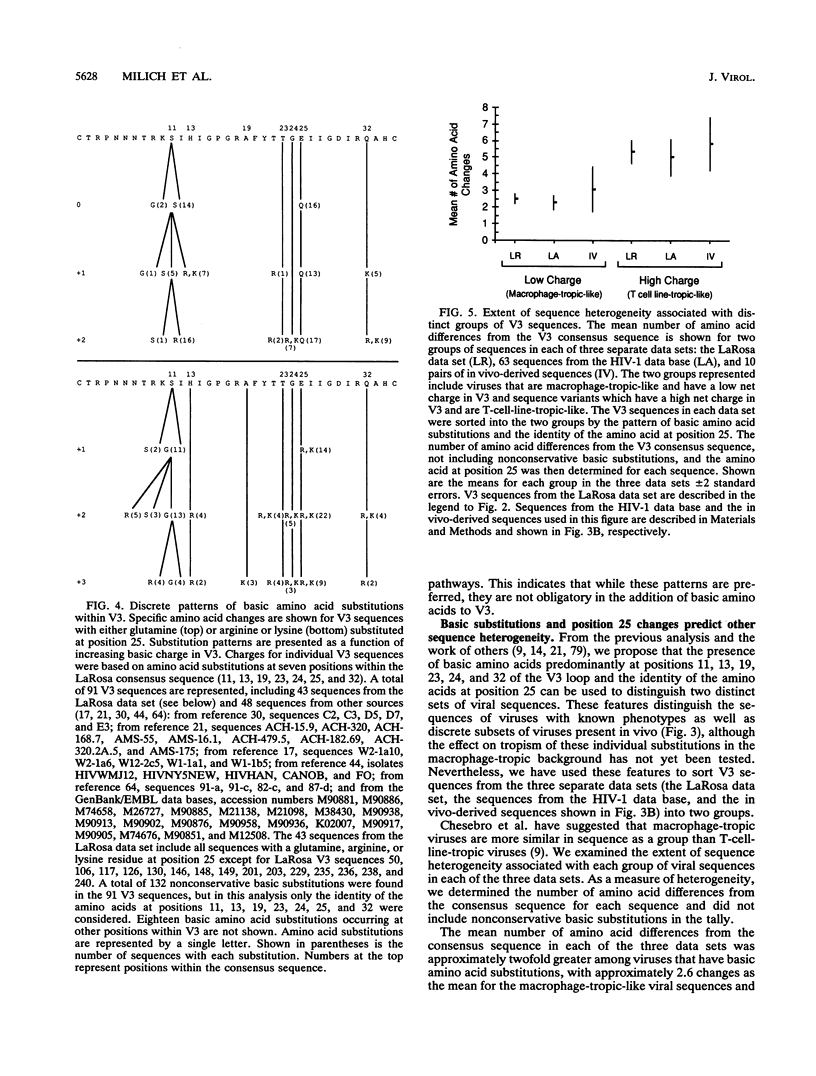
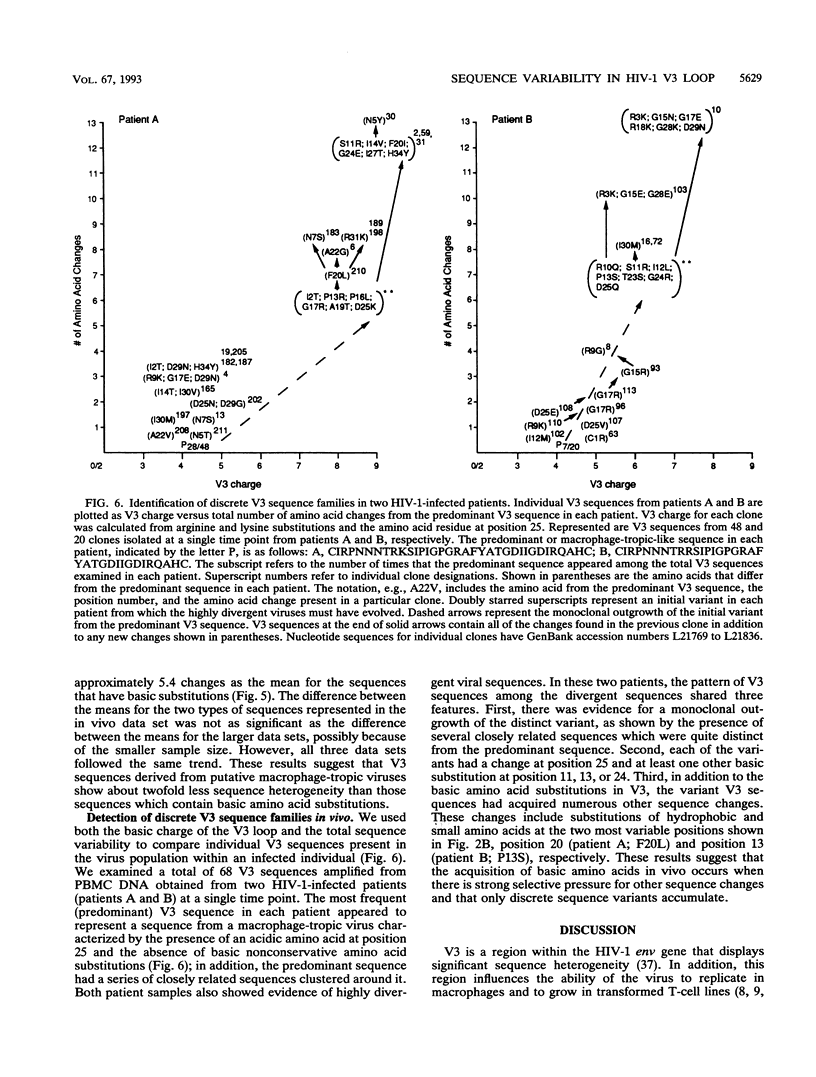
Two different states of human immunodeficiency virus type 1 are apparent in the asymptomatic and late stages of infection. Important determinants associated with these two states have been found within the V3 loop of the viral Env protein. In this study, two large data sets of published V3 sequences were analyzed to identify patterns of sequence variability that would correspond to these two states of the virus. We were especially interested in the pattern of basic amino acid substitutions, since the presence of basic amino acids in V3 has been shown to change virus tropism in cell culture. Four features of the sequence heterogeneity in V3 were observed: (i) approximately 70% of all nonconservative basic substitutions occur at four positions in V3, and V3 sequences with a basic substitution in at least one of these four positions contain approximately 95% of all nonconservative basic substitutions; (ii) substitution patterns within V3 are influenced by the identity of the amino acid at position 25; (iii) sequence polymorphisms account for a significant fraction of uncharged amino acid substitutions at several positions in V3, and sequence heterogeneity other than these polymorphisms is most significant at two positions near the tip of V3; and (iv) sequence heterogeneity in V3 (in addition to the basic amino acid substitutions) is approximately twofold greater in V3 sequences that contain basic amino acid substitutions. By using this sequence analysis, we were able to identify distinct groups of V3 sequences in infected patients that appear to correspond to these two virus states. The identification of these discrete sequence patterns in vivo demonstrates how the V3 sequence can be used as a genetic marker for studying the two states of human immunodeficiency virus type 1.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Cann A. J., Churcher M. J., Boyd M., O'Brien W., Zhao J. Q., Zack J., Chen I. S. The region of the envelope gene of human immunodeficiency virus type 1 responsible for determination of cell tropism. J Virol. 1992 Jan;66(1):305–309. doi: 10.1128/jvi.66.1.305-309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar K., Profy A. T., Dyson H. J. Solution conformational preferences of immunogenic peptides derived from the principal neutralizing determinant of the HIV-1 envelope glycoprotein gp120. Biochemistry. 1991 Sep 24;30(38):9187–9194. doi: 10.1021/bi00102a009. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Homsy J., Evans L. A., Levy J. A. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2815–2819. doi: 10.1073/pnas.85.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Nishio J., Perryman S., Cann A., O'Brien W., Chen I. S., Wehrly K. Identification of human immunodeficiency virus envelope gene sequences influencing viral entry into CD4-positive HeLa cells, T-leukemia cells, and macrophages. J Virol. 1991 Nov;65(11):5782–5789. doi: 10.1128/jvi.65.11.5782-5789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Bernstein D. C., Mann D. L., Shearer G. M., Berzofsky J. A. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989 Jun 1;339(6223):383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Crowl R., Ganguly K., Gordon M., Conroy R., Schaber M., Kramer R., Shaw G., Wong-Staal F., Reddy E. P. HTLV-III env gene products synthesized in E. coli are recognized by antibodies present in the sera of AIDS patients. Cell. 1985 Jul;41(3):979–986. doi: 10.1016/s0092-8674(85)80078-7. [DOI] [PubMed] [Google Scholar]
- De Jong J. J., De Ronde A., Keulen W., Tersmette M., Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992 Nov;66(11):6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. G., Kuiken C., Blumberg B. M., Hartman S., Sharer L. R., Clement M., Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991 Feb;180(2):583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Evans L. A., McHugh T. M., Stites D. P., Levy J. A. Differential ability of human immunodeficiency virus isolates to productively infect human cells. J Immunol. 1987 May 15;138(10):3415–3418. [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Back N. K., Nara P. L. Genomic diversity and antigenic variation of HIV-1: links between pathogenesis, epidemiology and vaccine development. FASEB J. 1991 Jul;5(10):2427–2436. doi: 10.1096/fasebj.5.10.2065891. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Guo H. G., Franchini G., Aldovini A., Collalti E., Farrell K., Wong-Staal F., Gallo R. C., Reitz M. S., Jr Envelope sequences of two new United States HIV-1 isolates. Virology. 1988 Jun;164(2):531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. C., Zhang L. Q., Simmonds P., Ludlam C. A., Brown A. J. Convergent and divergent sequence evolution in the surface envelope glycoprotein of human immunodeficiency virus type 1 within a single infected patient. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4835–4839. doi: 10.1073/pnas.89.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W. R., Matthews T. J., Ganfield M. C., Langlois A. J., Waselefsky D. M., Petteway S. R., Jr Antibodies from human immunodeficiency virus-infected individuals bind to a short amino acid sequence that elicits neutralizing antibodies in animals. AIDS Res Hum Retroviruses. 1989 Apr;5(2):173–182. doi: 10.1089/aid.1989.5.173. [DOI] [PubMed] [Google Scholar]
- Kuiken C. L., de Jong J. J., Baan E., Keulen W., Tersmette M., Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Jul;66(7):4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C. L., de Jong J. J., Baan E., Keulen W., Tersmette M., Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Sep;66(9):5704–5704. [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant: corrections and clarifications. Science. 1991 Feb 15;251(4995):811–811. doi: 10.1126/science.1990444. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Weinhold K., Profy A. T., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P., Bolognesi D. P., Matthews T. J., Emini E. A. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant: further clarifications. Science. 1991 Sep 6;253(5024):1146–1146. doi: 10.1126/science.1887238. [DOI] [PubMed] [Google Scholar]
- Liu Z. Q., Wood C., Levy J. A., Cheng-Mayer C. The viral envelope gene is involved in macrophage tropism of a human immunodeficiency virus type 1 strain isolated from brain tissue. J Virol. 1990 Dec;64(12):6148–6153. doi: 10.1128/jvi.64.12.6148-6153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Hornickova Z., Markham R., Birdwell A., Arens M., Saah A., Ratner L. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10247–10251. doi: 10.1073/pnas.89.21.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Garrity R. R., Goudsmit J. Neutralization of HIV-1: a paradox of humoral proportions. FASEB J. 1991 Jul;5(10):2437–2455. doi: 10.1096/fasebj.5.10.1712328. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Ciesielski C. A., Myers G., Bandea C. I., Luo C. C., Korber B. T., Mullins J. I., Schochetman G., Berkelman R. L., Economou A. N. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992 May 22;256(5060):1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Matthews T. J., Langlois A., Tanner M. E., Martin M. E., Scearce R. M., Kim J. E., Berzofsky J. A., Bolognesi D. P., Haynes B. F. Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J Immunol. 1989 May 15;142(10):3612–3619. [PubMed] [Google Scholar]
- Rabson A. B., Martin M. A. Molecular organization of the AIDS retrovirus. Cell. 1985 Mar;40(3):477–480. doi: 10.1016/0092-8674(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Roos M. T., Lange J. M., de Goede R. E., Coutinho R. A., Schellekens P. T., Miedema F., Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992 Mar;165(3):427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Sauermann U., Schneider J., Mous J., Brunckhorst U., Schedel I., Jentsch K. D., Hunsmann G. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res Hum Retroviruses. 1990 Jun;6(6):813–823. doi: 10.1089/aid.1990.6.813. [DOI] [PubMed] [Google Scholar]
- Scarlatti G., Leitner T., Halapi E., Wahlberg J., Marchisio P., Clerici-Schoeller M. A., Wigzell H., Fenyö E. M., Albert J., Uhlén M. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1721–1725. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Koot M., Kootstra N. A., Dercksen M. W., de Goede R. E., van Steenwijk R. P., Lange J. M., Schattenkerk J. K., Miedema F., Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992 Mar;66(3):1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Kootstra N. A., de Goede R. E., de Wolf F., Miedema F., Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991 Jan;65(1):356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Fenyö E. M., Pavlakis G. N. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7200–7203. doi: 10.1073/pnas.86.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., McOmish F., Balfe P., Ludlam C. A., Brown A. J. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J Virol. 1991 Nov;65(11):6266–6276. doi: 10.1128/jvi.65.11.6266-6276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six H. R., Webster R. G., Kendal A. P., Glezen W. P., Griffis C., Couch R. B. Antigenic analysis of H1N1 viruses isolated in the Houston metropolitan area during four successive seasons. Infect Immun. 1983 Nov;42(2):453–458. doi: 10.1128/iai.42.2.453-458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Langlois A. J., McDanal C. B., McDougal J. S., Bolognesi D. P., Matthews T. J. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J Virol. 1988 Nov;62(11):4195–4200. doi: 10.1128/jvi.62.11.4195-4200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Germain R. N., Moss B., Berzofsky J. A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 class II-restricted help for itself. J Exp Med. 1990 Feb 1;171(2):571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakagawa Y., Pendleton C. D., Houghten R. A., Yokomuro K., Germain R. N., Berzofsky J. A. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992 Jan 17;255(5042):333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Akutsu M., Murayama K., Shimizu N., Hoshino H. Host range mutant of human immunodeficiency virus type 1: modification of cell tropism by a single point mutation at the neutralization epitope in the env gene. J Virol. 1991 Apr;65(4):1710–1718. doi: 10.1128/jvi.65.4.1710-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., de Goede R. E., Al B. J., Winkel I. N., Gruters R. A., Cuypers H. T., Huisman H. G., Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Kendal A. P., Gerhard W. Analysis of antigenic drift in recently isolated influenza A (H1N1) viruses using monoclonal antibody preparations. Virology. 1979 Jul 15;96(1):258–264. doi: 10.1016/0042-6822(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- York-Higgins D., Cheng-Mayer C., Bauer D., Levy J. A., Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990 Aug;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- Zvi A., Hiller R., Anglister J. Solution conformation of a peptide corresponding to the principal neutralizing determinant of HIV-1IIIB: a two-dimensional NMR study. Biochemistry. 1992 Aug 4;31(30):6972–6979. doi: 10.1021/bi00145a015. [DOI] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



