Abstract
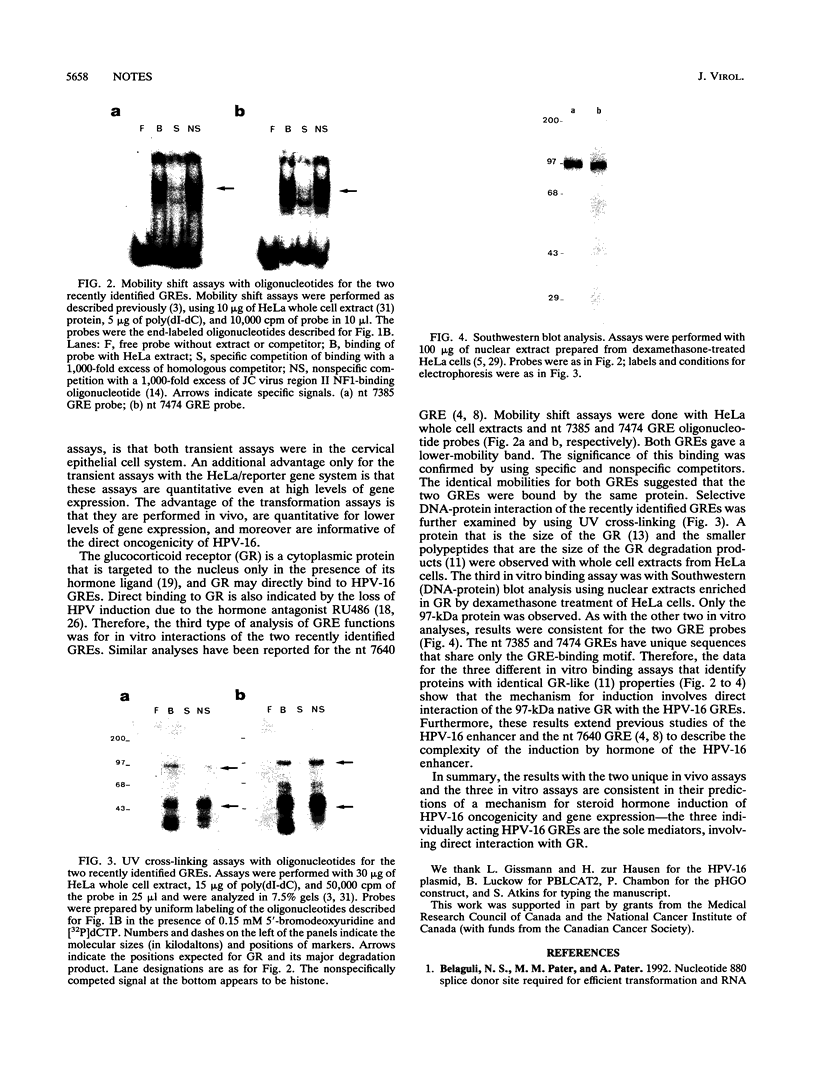
We have previously shown that human papillomavirus type 16 (HPV-16) can efficiently transform primary baby rat kidney cells in the presence of the steroid hormones progesterone and the glucocorticoid dexamethasone. To study this effect of hormone, different combinations of the previously identified glucocorticoid response element (GRE) at nucleotide 7640 of HPV-16 and the other two GREs that we have recently identified, at nucleotides 7385 and 7474, were mutated. The previously described GRE and the other two GREs were shown to be functional for the induction of transformation by dexamethasone. In addition, transient assays in cervical HeLa cells demonstrated the functional importance of the three individual GREs. Assays for in vitro interaction demonstrated the specific binding of a 97-kDa protein, the glucocorticoid receptor, to both recently identified HPV-16 GREs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belaguli N. S., Pater M. M., Pater A. Nucleotide 880 splice donor site required for efficient transformation and RNA accumulation by human papillomavirus type 16 E7 gene. J Virol. 1992 May;66(5):2724–2730. doi: 10.1128/jvi.66.5.2724-2730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton L. A., Reeves W. C., Brenes M. M., Herrero R., de Britton R. C., Gaitan E., Tenorio F., Garcia M., Rawls W. E. Oral contraceptive use and risk of invasive cervical cancer. Int J Epidemiol. 1990 Mar;19(1):4–11. doi: 10.1093/ije/19.1.4. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Klock G., Bernard H. U. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J Virol. 1989 Aug;63(8):3261–3269. doi: 10.1128/jvi.63.8.3261-3269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gallahan D., Jay G., Rhim J. S. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989 Dec;173(2):767–771. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Gitsch G., Kainz C., Studnicka M., Reinthaller A., Tatra G., Breitenecker G. Oral contraceptives and human papillomavirus infection in cervical intraepithelial neoplasia. Arch Gynecol Obstet. 1992;252(1):25–30. doi: 10.1007/BF02389603. [DOI] [PubMed] [Google Scholar]
- Gloss B., Bernard H. U., Seedorf K., Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987 Dec 1;6(12):3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Chong T., Bernard H. U. Numerous nuclear proteins bind the long control region of human papillomavirus type 16: a subset of 6 of 23 DNase I-protected segments coincides with the location of the cell-type-specific enhancer. J Virol. 1989 Mar;63(3):1142–1152. doi: 10.1128/jvi.63.3.1142-1152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan M. V., Gronemeyer H. Characterization of the rat liver glucocorticoid receptor purified by DNA-cellulose and ligand affinity chromatography. J Biol Chem. 1984 Oct 25;259(20):12915–12924. [PubMed] [Google Scholar]
- Hildesheim A., Reeves W. C., Brinton L. A., Lavery C., Brenes M., De La Guardia M. E., Godoy J., Rawls W. E. Association of oral contraceptive use and human papillomaviruses in invasive cervical cancers. Int J Cancer. 1990 May 15;45(5):860–864. doi: 10.1002/ijc.2910450513. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. U., Pater A., Pater M. M. Human JC virus perfect palindromic nuclear factor 1-binding sequences important for glial cell-specific expression in differentiating embryonal carcinoma cells. J Virol. 1993 Jan;67(1):572–576. doi: 10.1128/jvi.67.1.572-576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Kyo S., Inoue M., Nishio Y., Nakanishi K., Akira S., Inoue H., Yutsudo M., Tanizawa O., Hakura A. NF-IL6 represses early gene expression of human papillomavirus type 16 through binding to the noncoding region. J Virol. 1993 Feb;67(2):1058–1066. doi: 10.1128/jvi.67.2.1058-1066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Tsutsumi K., Pater A., Pater M. M. Human papillomavirus type 16 expression in cervical keratinocytes: role of progesterone and glucocorticoid hormones. Obstet Gynecol. 1993 Jan;81(1):5–12. [PubMed] [Google Scholar]
- Muller M., Renkawitz R. The glucocorticoid receptor. Biochim Biophys Acta. 1991 Feb 16;1088(2):171–182. doi: 10.1016/0167-4781(91)90052-n. [DOI] [PubMed] [Google Scholar]
- Nakshatri H., Pater M. M., Pater A. Ubiquitous and cell-type-specific protein interactions with human papillomavirus type 16 and type 18 enhancers. Virology. 1990 Sep;178(1):92–103. doi: 10.1016/0042-6822(90)90382-2. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Suh B. J., Kühnel B., Hutchison C. A., 3rd Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990 Dec;4(12):1866–1873. doi: 10.1210/mend-4-12-1866. [DOI] [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Pater A., Bayatpour M., Pater M. M. Oncogenic transformation by human papillomavirus type 16 deoxyribonucleic acid in the presence of progesterone or progestins from oral contraceptives. Am J Obstet Gynecol. 1990 Apr;162(4):1099–1103. doi: 10.1016/0002-9378(90)91323-5. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Hughes G. A., Hyslop D. E., Nakshatri H., Pater A. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988 Oct 27;335(6193):832–835. doi: 10.1038/335832a0. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Pater A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology. 1985 Sep;145(2):313–318. doi: 10.1016/0042-6822(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Pater A. RU486 inhibits glucocorticoid hormone-dependent oncogenesis by human papillomavirus type 16 DNA. Virology. 1991 Aug;183(2):799–802. doi: 10.1016/0042-6822(91)91014-8. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Phelps W. C., Zhang Y. L., Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 Oct;7(10):3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Hotz M., Gissmann L. Increased prevalence of human papillomaviruses in the lower genital tract of pregnant women. Int J Cancer. 1987 Aug 15;40(2):198–201. doi: 10.1002/ijc.2910400212. [DOI] [PubMed] [Google Scholar]
- Silva C. M., Tully D. B., Petch L. A., Jewell C. M., Cidlowski J. A. Application of a protein-blotting procedure to the study of human glucocorticoid receptor interactions with DNA. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1744–1748. doi: 10.1073/pnas.84.7.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E., Forsythe A. B., Youkeles L., Coffelt C. F. Steroid contraceptive use and cervical dysplasia: increased risk of progression. Science. 1977 Jun 24;196(4297):1460–1462. doi: 10.1126/science.867043. [DOI] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]