Abstract
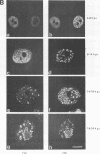
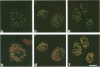
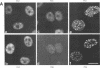
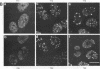
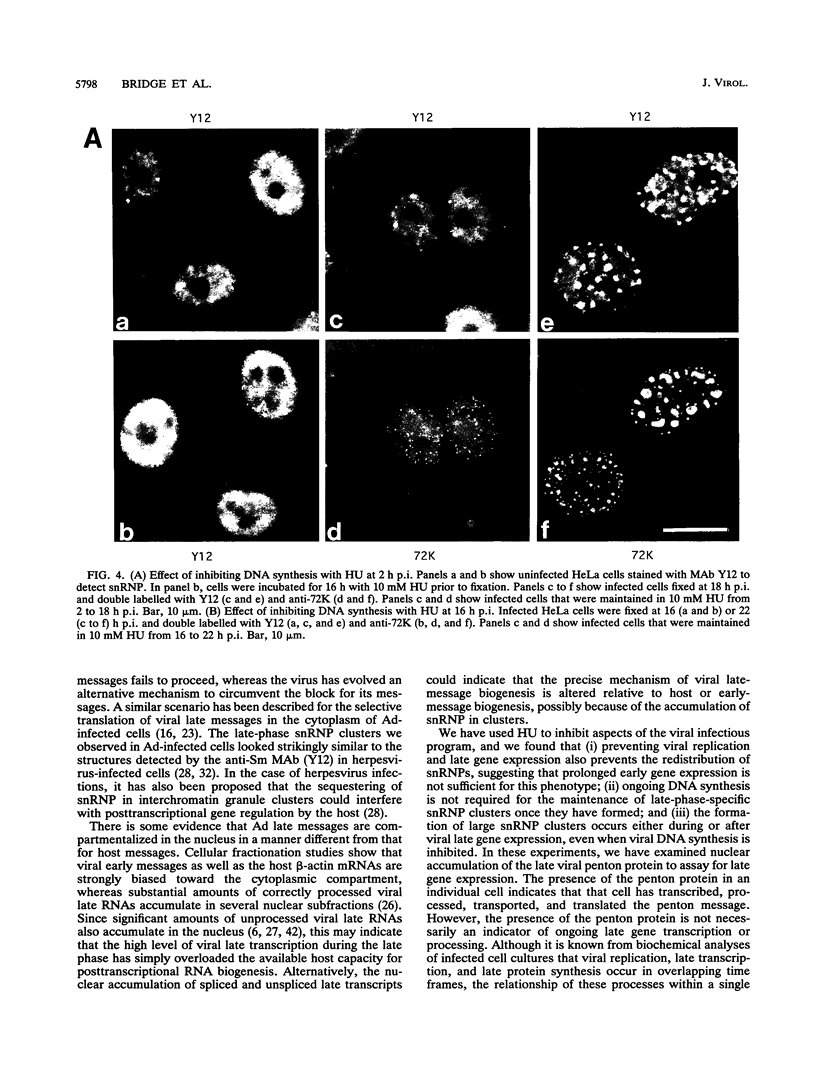
We have studied the effect of adenovirus infection on the nuclear organization of splicing small nuclear ribonucleoproteins (snRNPs) in HeLa cells. In uninfected HeLa cells, snRNPs are widespread throughout the nucleoplasm but also are concentrated in specific nuclear structures, including coiled bodies, interchromatin granules, and perichromatin fibrils. We have used immunofluorescence microscopy to study the localization of splicing snRNPs relative to centers of viral DNA synthesis and accumulation identified with antiserum against the viral 72,000-molecular-weight single-stranded DNA-binding protein (72K protein). Splicing snRNPs were independently detected with both monoclonal and polyclonal antibodies specific for common snRNP antigens, snRNP-specific proteins, and the snRNA-specific 2,2,7-trimethylguanosine 5' cap structure. We have examined infected cells 2 to 24 h after infection, and, in the majority of these cells, we observed no colocalization of the snRNP and 72K-protein staining patterns. In the late phase, snRNPs were found to markedly concentrate in discrete clusters that were distinct from the centers of viral DNA synthesis and accumulation identified with anti-72K protein. We have treated cells with hydroxyurea at various times after infection to inhibit aspects of the virus infectious program. We have found that the accumulation of snRNP clusters is correlated with late gene expression rather than with DNA synthesis or early gene expression. Finally, we show that the late-phase snRNP clusters colocalize with a monoclonal antibody that primarily stains interchromatin granules. These results suggest that the centers of snRNP concentration in late-phase infected cells are likely to correspond to interchromatin granule clusters.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1987 Oct;61(10):3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M., Sharp P. A. Structure of late adenovirus 2 heterogeneous nuclear RNA. J Mol Biol. 1979 Apr 25;129(4):547–565. doi: 10.1016/0022-2836(79)90468-6. [DOI] [PubMed] [Google Scholar]
- Bosher J., Dawson A., Hay R. T. Nuclear factor I is specifically targeted to discrete subnuclear sites in adenovirus type 2-infected cells. J Virol. 1992 May;66(5):3140–3150. doi: 10.1128/jvi.66.5.3140-3150.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Ferreira J., Lamond A. I. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993 Feb;120(4):841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Pepperkok R., Barabino S. M., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991 Jan;10(1):195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Taneja K. L., Lawrence J. B. Discrete nuclear domains of poly(A) RNA and their relationship to the functional organization of the nucleus. J Cell Biol. 1991 Dec;115(5):1191–1202. doi: 10.1083/jcb.115.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Cleghon V. G., Klessig D. F. Association of the adenovirus DNA-binding protein with RNA both in vitro and in vivo. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8947–8951. doi: 10.1073/pnas.83.23.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Racaniello V., Villamarin A., Palladino F., Schneider R. J. The adenovirus tripartite leader may eliminate the requirement for cap-binding protein complex during translation initiation. J Virol. 1988 Jun;62(6):2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Steitz J. A. A protein associated with small nuclear ribonucleoprotein particles recognizes the 3' splice site of premessenger RNA. Cell. 1986 Dec 26;47(6):973–984. doi: 10.1016/0092-8674(86)90812-3. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., De Jong B. A., Van der Kemp A., Van Venrooij W. J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989 Oct 15;143(8):2560–2566. [PubMed] [Google Scholar]
- Hodge L. D., Scharff M. D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969 Apr;37(4):554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991 Apr 19;65(2):271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Leppard K. N., Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989 Aug;8(8):2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: identification and comparison of adenovirus 2 encoded transcripts synthesized in vitro and vivo. J Mol Biol. 1979 Nov 25;135(1):171–197. doi: 10.1016/0022-2836(79)90346-2. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Barghusen S. C., Leser G. P., Spear P. G. Redistribution of nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987 Nov;105(5):2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman U., Hallman H., Hadlaczky G., Pettersson I., Sharp G., Ringertz N. R. Intranuclear localization of snRNP antigens. J Cell Biol. 1986 Jan;102(1):137–144. doi: 10.1083/jcb.102.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Konarska M. M., Grabowski P. J., Hardy S. F., Sharp P. A. Lariat RNA's as intermediates and products in the splicing of messenger RNA precursors. Science. 1984 Aug 31;225(4665):898–903. doi: 10.1126/science.6206566. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Puvion E. Sites of transcription of adenovirus type 5 genomes in relation to early viral DNA replication in infected HeLa cells. A high resolution in situ hybridization and autoradiographical study. Biol Cell. 1991;71(1-2):135–147. doi: 10.1016/0248-4900(91)90060-z. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Roussev R., Puvion E. Distribution of viral RNA molecules during the adenovirus type 5 infectious cycle in HeLa cells. J Struct Biol. 1992 May-Jun;108(3):209–220. doi: 10.1016/1047-8477(92)90021-2. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Andrade L. E., Chan E. K., Burlingame R., Peebles C., Gruol D., Tan E. M. Association between the nucleolus and the coiled body. J Struct Biol. 1990 Jul-Sep;104(1-3):120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Reuter R., Appel B., Bringmann P., Rinke J., Lührmann R. 5'-Terminal caps of snRNAs are reactive with antibodies specific for 2,2,7-trimethylguanosine in whole cells and nuclear matrices. Double-label immunofluorescent studies with anti-m3G antibodies and with anti-RNP and anti-Sm autoantibodies. Exp Cell Res. 1984 Oct;154(2):548–560. doi: 10.1016/0014-4827(84)90179-4. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sandler A. B., Ketner G. The metabolism of host RNAs in cells infected by an adenovirus E4 mutant. Virology. 1991 Mar;181(1):319–326. doi: 10.1016/0042-6822(91)90498-z. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Davies S., Whitfield W. G. Characterization of a family of nuclear and chromosomal proteins identified by a monoclonal antibody. Eur J Cell Biol. 1985 Sep;38(2):344–352. [PubMed] [Google Scholar]
- Turner B. M., Franchi L. Identification of protein antigens associated with the nuclear matrix and with clusters of interchromatin granules in both interphase and mitotic cells. J Cell Sci. 1987 Mar;87(Pt 2):269–282. doi: 10.1242/jcs.87.2.269. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. H., Moen P. T., Jr, Fox E., Bodnar J. W. Interactions of minute virus of mice and adenovirus with host nucleoli. J Virol. 1989 Sep;63(9):3651–3660. doi: 10.1128/jvi.63.9.3651-3660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cao L. G., Wang Y. L., Pederson T. Localization of pre-messenger RNA at discrete nuclear sites. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7391–7395. doi: 10.1073/pnas.88.16.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Hsu M. T. Visualization of nascent RNA transcripts and simultaneous transcription and replication in viral nucleoprotein complexes from adenovirus 2-infected HeLa cells. J Mol Biol. 1981 Apr 5;147(2):247–268. doi: 10.1016/0022-2836(81)90440-x. [DOI] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Dobner P. R., Lawrence J. B. Higher level organization of individual gene transcription and RNA splicing. Science. 1993 Feb 26;259(5099):1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991 Jan;10(1):207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zamore P. D., Carmo-Fonseca M., Lamond A. I., Green M. R. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8769–8773. doi: 10.1073/pnas.89.18.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]