Abstract
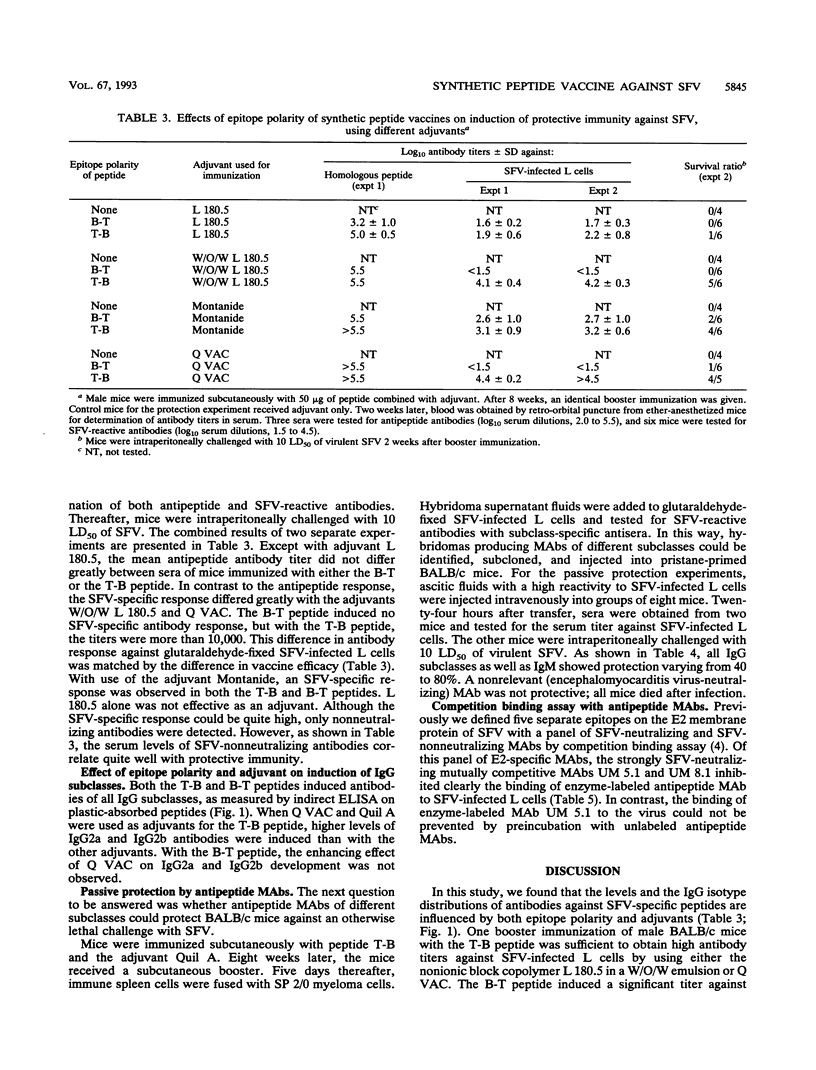
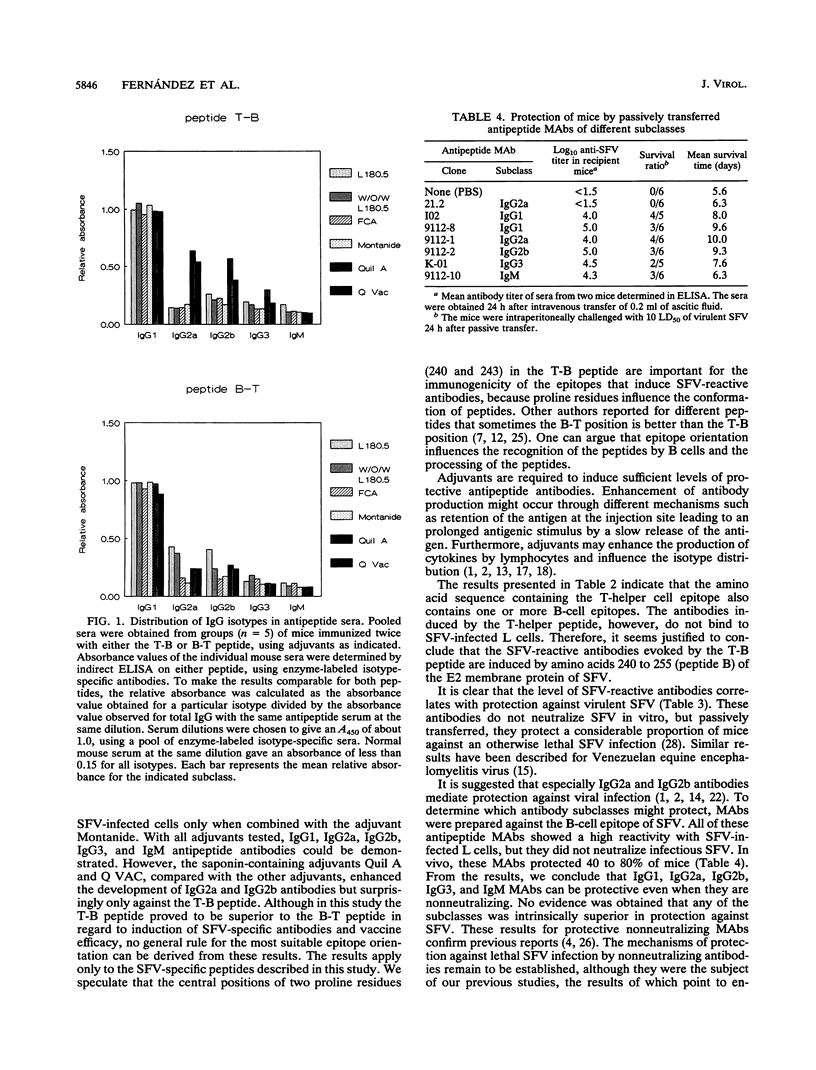
The antibody response to a previously defined B-cell epitope of Semliki Forest virus (SFV) was investigated in male BALB/c (H-2d) mice. The B-cell epitope, located at amino acid positions 240 to 255 of the E2 protein, was linked to an H-2d-restricted T-helper cell epitope of SFV located at positions 137 to 151 of the E2 protein. Colinearly synthesized peptides, of either T-B or B-T polarity, mixed with different adjuvants (the nonionic block copolymer L 180.5, a water-oil-water [W/O/W] emulsion of L 180.5, Montanide, and Q VAC) were used for immunization. Generally, after one booster immunization, high serum antibody titers were measured against either peptide. With Q VAC and W/O/W L 180.5 as adjuvants, the titers of SFV-reactive (nonneutralizing) antibodies were consistently much higher after immunization with the T-B peptide than with the B-T peptide, which was reflected in a higher vaccine efficacy. With these two adjuvants, the survival ratio in T-B peptide-immunized mice was 82%, compared with 8% in B-T peptide-immunized mice. Intermediate results were obtained with the adjuvant Montanide. L 180.5 alone was ineffective in this study. All immunoglobulin G (IgG) isotypes were induced with either adjuvant, but Q VAC was clearly the most effective in inducing IgG2a and IgG2b isotypes with the T-B peptide as the antigen. Subsequently, monoclonal antibodies (MAbs) of IgM, IgG1, IgG2a, IgG2b, and IgG3 subclasses were prepared against the B-cell epitope. These nonneutralizing but SFV-reactive MAbs protected 40 to 80% of mice against a lethal challenge with SFV. Control mice all died. The availability of those antipeptide MAbs allowed competition binding assays with a previously characterized panel of E2-specific MAbs. Binding of enzyme-labeled antipeptide MAbs was very effectively inhibited by two strongly SFV-neutralizing mutually competitive MAbs, suggesting that the linear B-cell epitope (amino acids 240 to 255) is associated with a major neutralization site of SFV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Byars N. E. Immunological adjuvants: desirable properties and side-effects. Mol Immunol. 1991 Mar;28(3):279–284. doi: 10.1016/0161-5890(91)90074-t. [DOI] [PubMed] [Google Scholar]
- Barteling S. J., Vreeswijk J. Developments in foot-and-mouth disease vaccines. Vaccine. 1991 Feb;9(2):75–88. doi: 10.1016/0264-410x(91)90261-4. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J Virol. 1985 May;54(2):546–551. doi: 10.1128/jvi.54.2.546-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Ivanyi J., Young D. B., Lamb J. R., Syred A. D., Francis M. J. Orientation of epitopes influences the immunogenicity of synthetic peptide dimers. Eur J Immunol. 1988 Dec;18(12):2015–2019. doi: 10.1002/eji.1830181222. [DOI] [PubMed] [Google Scholar]
- Dalsgaard K. Adjuvants. Vet Immunol Immunopathol. 1987 Dec;17(1-4):145–152. doi: 10.1016/0165-2427(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Faragher S. G., Meek A. D., Rice C. M., Dalgarno L. Genome sequences of a mouse-avirulent and a mouse-virulent strain of Ross River virus. Virology. 1988 Apr;163(2):509–526. doi: 10.1016/0042-6822(88)90292-9. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Golvano J., Lasarte J. J., Sarobe P., Gullón A., Prieto J., Borrás-Cuesta F. Polarity of immunogens: implications for vaccine design. Eur J Immunol. 1990 Oct;20(10):2363–2366. doi: 10.1002/eji.1830201031. [DOI] [PubMed] [Google Scholar]
- Hadjipetrou-Kourounakis L., Möller E. Adjuvants influence the immunoglobin subclass distribution of immune responses in vivo. Scand J Immunol. 1984 Mar;19(3):219–225. doi: 10.1111/j.1365-3083.1984.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Hocart M. J., Mackenzie J. S., Stewart G. A. The immunoglobulin G subclass responses of mice to influenza A virus: the effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J Gen Virol. 1989 Sep;70(Pt 9):2439–2448. doi: 10.1099/0022-1317-70-9-2439. [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Short W. A., Johnson A. J., Bolin R. A., Roehrig J. T. Synthetic peptides of the E2 glycoprotein of Venezuelan equine encephalomyelitis virus. II. Antibody to the amino terminus protects animals by limiting viral replication. Virology. 1991 Nov;185(1):281–290. doi: 10.1016/0042-6822(91)90775-7. [DOI] [PubMed] [Google Scholar]
- Hunter R., Olsen M., Buynitzky S. Adjuvant activity of non-ionic block copolymers. IV. Effect of molecular weight and formulation on titre and isotype of antibody. Vaccine. 1991 Apr;9(4):250–256. doi: 10.1016/0264-410x(91)90108-i. [DOI] [PubMed] [Google Scholar]
- Karagouni E. E., Hadjipetrou-Kourounakis L. Regulation of isotype immunoglobulin production by adjuvants in vivo. Scand J Immunol. 1990 Jun;31(6):745–754. doi: 10.1111/j.1365-3083.1990.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Kenney J. S., Hughes B. W., Masada M. P., Allison A. C. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J Immunol Methods. 1989 Jul 26;121(2):157–166. doi: 10.1016/0022-1759(89)90156-7. [DOI] [PubMed] [Google Scholar]
- Kensil C. R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991 Jan 15;146(2):431–437. [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- McKendall R. R., Woo W. Murine IgG subclass responses to herpes simplex virus type 1 and polypeptides. J Gen Virol. 1988 Apr;69(Pt 4):847–857. doi: 10.1099/0022-1317-69-4-847. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Oosterlaken T. A., Vlaspolder F., Fransen R., Harmsen T., Kraaijeveld C. A., Snippe H. Competition binding assay in cell culture for identification of epitopes on enveloped and naked viruses. Zentralbl Bakteriol. 1989 Jul;271(2):237–243. doi: 10.1016/s0934-8840(89)80078-7. [DOI] [PubMed] [Google Scholar]
- Partidos C., Stanley C., Steward M. The influence of orientation and number of copies of T and B cell epitopes on the specificity and affinity of antibodies induced by chimeric peptides. Eur J Immunol. 1992 Oct;22(10):2675–2680. doi: 10.1002/eji.1830221030. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Snijders A., Benaissa-Trouw B. J., Oosterlaken T. A., Puijk W. C., Posthumus W. P., Meloen R. H., Boere W. A., Oosting J. D., Kraaijeveld C. A., Snippe H. Identification of linear epitopes on Semliki Forest virus E2 membrane protein and their effectiveness as a synthetic peptide vaccine. J Gen Virol. 1991 Mar;72(Pt 3):557–565. doi: 10.1099/0022-1317-72-3-557. [DOI] [PubMed] [Google Scholar]
- Snijders A., Benaissa-Trouw B. J., Snippe H., Kraaijeveld C. A. Immunogenicity and vaccine efficacy of synthetic peptides containing Semliki Forest virus B and T cell epitopes. J Gen Virol. 1992 Sep;73(Pt 9):2267–2272. doi: 10.1099/0022-1317-73-9-2267. [DOI] [PubMed] [Google Scholar]
- Snijders A., Benaissa-Trouw B. J., Visser-Vernooy H. J., Fernandez I., Snippe H., Kraaijeveld C. A. A delayed-type hypersensitivity-inducing T-cell epitope of Semliki Forest virus mediates effective T-helper activity for antibody production. Immunology. 1992 Nov;77(3):322–329. [PMC free article] [PubMed] [Google Scholar]
- Vlaspolder F., Kraaijeveld C. A., van Buuren R., Harmsen M., Benaissa-Trouw B. J., Snippe H. Prophylaxis and therapy of virulent encephalomyocarditis virus infection in mice by monoclonal antibodies. Brief report. Arch Virol. 1988;98(1-2):123–130. doi: 10.1007/BF01321013. [DOI] [PubMed] [Google Scholar]
- Vrati S., Fernon C. A., Dalgarno L., Weir R. C. Location of a major antigenic site involved in Ross River virus neutralization. Virology. 1988 Feb;162(2):346–353. doi: 10.1016/0042-6822(88)90474-6. [DOI] [PubMed] [Google Scholar]



