Abstract
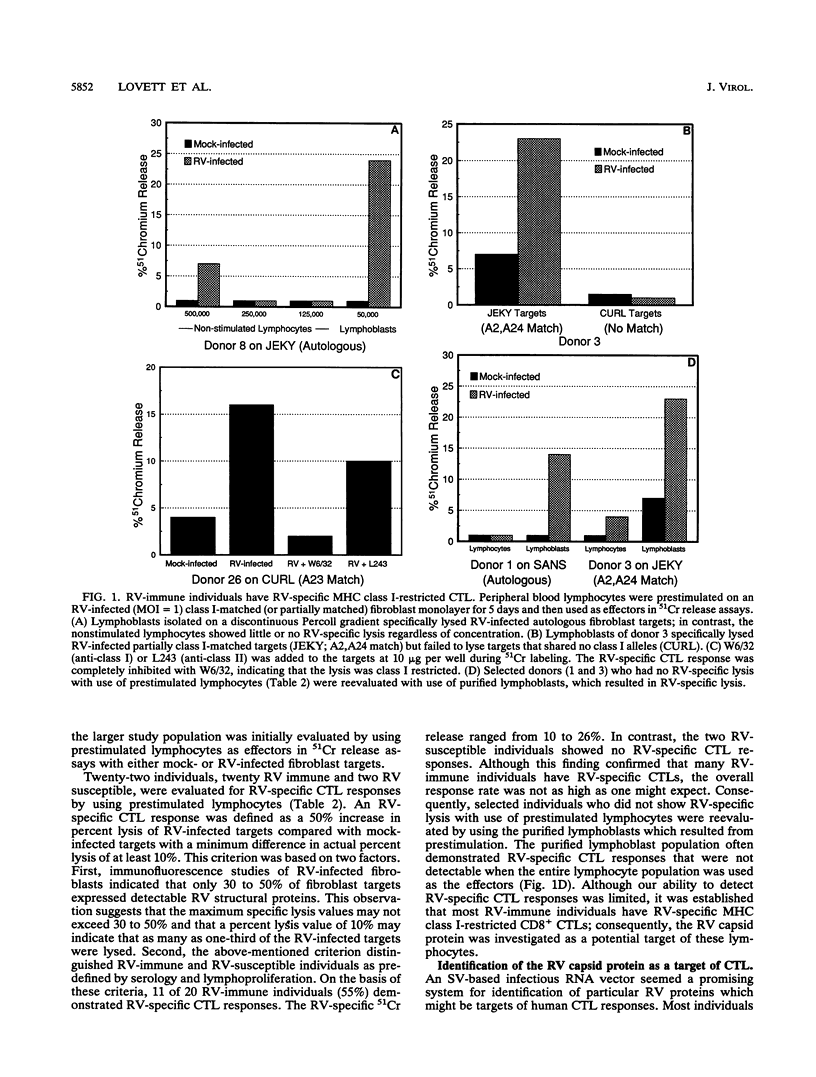
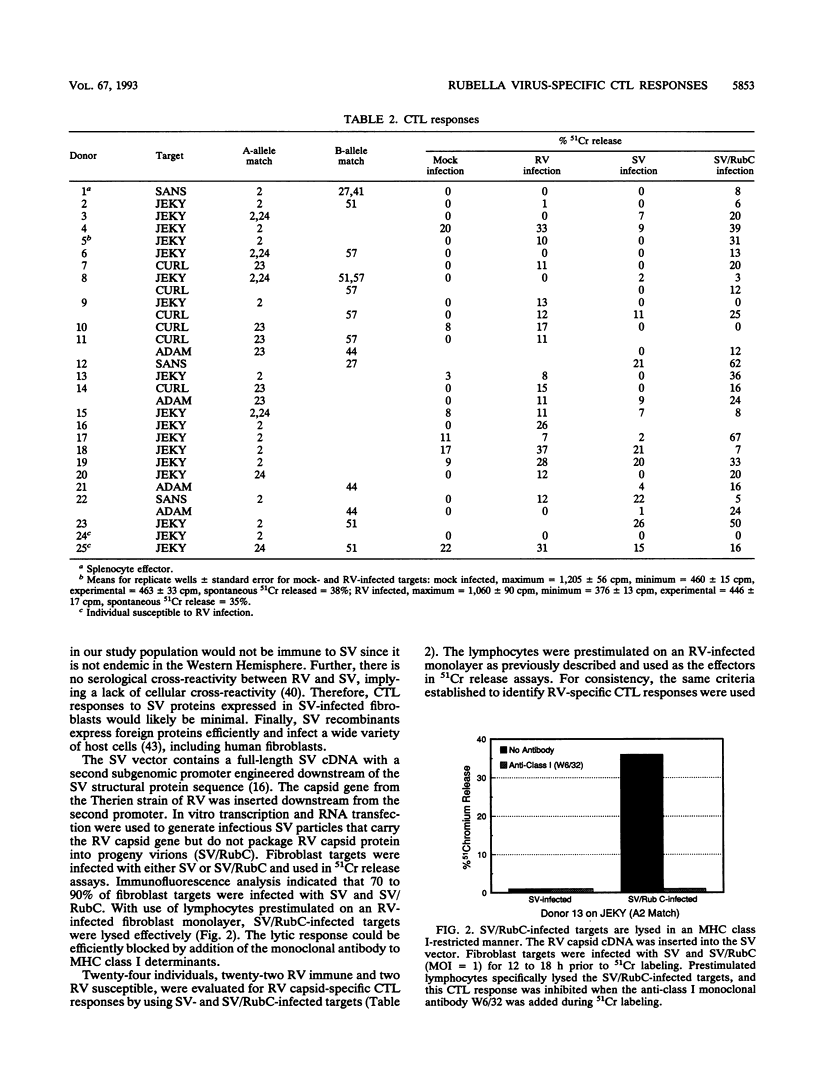
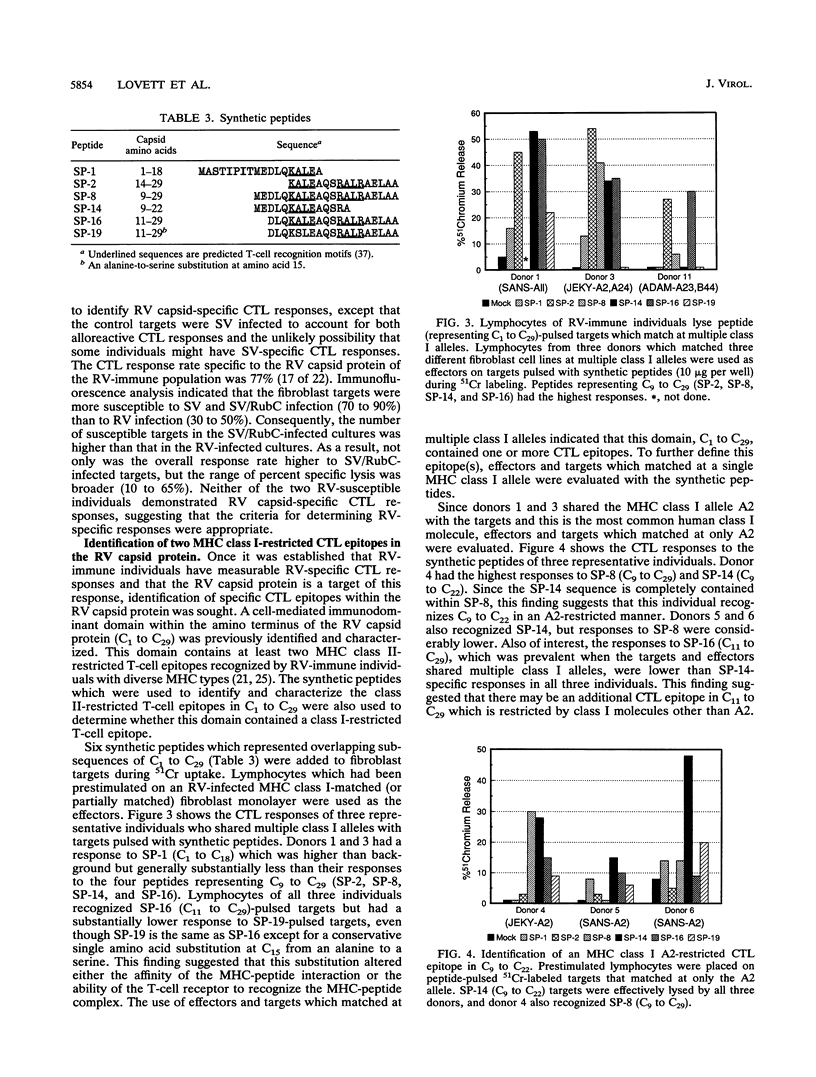
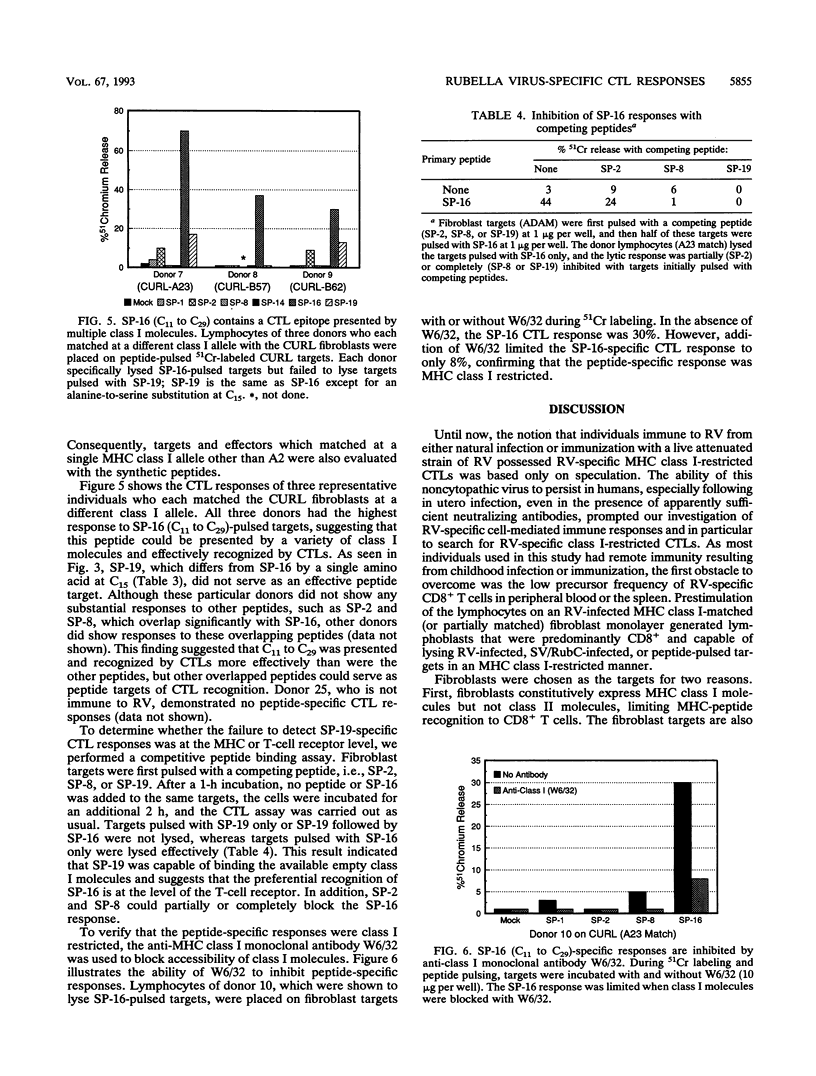
The role of major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T lymphocytes in immunity to rubella virus (RV) infection is unknown. Lymphocytes of RV-immune individuals were prestimulated on an RV-infected MHC class I-matched (or partially matched) fibroblast monolayer which generated CD8+ lymphoblasts capable of lysing RV-infected fibroblast targets in a class I-restricted manner. Using an infectious Sindbis virus (SV) vector which expressed the RV capsid protein (SV/RubC), lymphocytes from 17 of 22 RV-immune individuals prestimulated on RV-infected fibroblast monolayers lysed SV/RubC-infected fibroblast targets. A sequence within the amino terminus of the capsid protein that was previously shown to contain immunodominant class II-restricted T-cell epitopes was evaluated for class I-restricted epitopes. Fibroblast targets pulsed with synthetic peptides representing subsequences within C1 to C29 (subscripts indicate amino acid positions) were lysed effectively when the targets and effectors matched at multiple class I alleles. By limiting the number of matching class I alleles, an A2-restricted epitope was identified within C9 to C22 and an epitope that could be presented by multiple class I molecules was identified within C11 to C29. A sequence such as C1 to C29 which contains both MHC class I- and MHC class II-restricted epitopes recognized by a heterologous human population may serve as a component of an effective synthetic vaccine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Cooper L. Z. Cell-mediated immune response in rubella infections. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S123–S128. doi: 10.1093/clinids/7.supplement_1.s123. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Lang P. B., Ziring P. R., Cooper L. Z. Impaired cell-mediated immune response in patients with congenital rubella: correlation with gestational age at time of infection. Pediatrics. 1979 Nov;64(5):620–626. [PubMed] [Google Scholar]
- Buimovici-Klein E., Vesikari T., Santangelo C. F., Cooper L. Z. Study of the lymphocyte in vitro response to rubella antigen and phytohemagglutinin by a whole blood method. Arch Virol. 1976;52(4):323–331. doi: 10.1007/BF01315621. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Persistent rubella infection and rubella-associated arthritis. Lancet. 1982 Jun 12;1(8285):1323–1325. doi: 10.1016/s0140-6736(82)92398-4. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Rubella-associated arthritis: rescue of rubella virus from peripheral blood lymphocytes two years postvaccination. Infect Immun. 1981 Jun;32(3):1274–1280. doi: 10.1128/iai.32.3.1274-1280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler J. K., Tingle A. J., Petty R. E. Persistent rubella virus infection associated with chronic arthritis in children. N Engl J Med. 1985 Oct 31;313(18):1117–1123. doi: 10.1056/NEJM198510313131803. [DOI] [PubMed] [Google Scholar]
- Chaye H., Chong P., Tripet B., Brush B., Gillam S. Localization of the virus neutralizing and hemagglutinin epitopes of E1 glycoprotein of rubella virus. Virology. 1992 Aug;189(2):483–492. doi: 10.1016/0042-6822(92)90572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L. Z., Krugman S. Diagnosis and management: congenital rubella. Pediatrics. 1966 Feb;37(2):335–338. [PubMed] [Google Scholar]
- Cooper L. Z., Ziring P. R., Ockerse A. B., Fedun B. A., Kiely B., Krugman S. Rubella. Clinical manifestations and management. Am J Dis Child. 1969 Jul;118(1):18–29. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Frelinger J. A., Gotch F. M., Zweerink H., Wain E., McMichael A. J. Evidence of widespread binding of HLA class I molecules to peptides. J Exp Med. 1990 Sep 1;172(3):827–834. doi: 10.1084/jem.172.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Hahn Y. S., Braciale T. J., Rice C. M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanra G. Y., Vesikari T. Cytotoxic activity against rubella-infected cells in the supernatants of human lymphocyte cultures stimulated by rubella virus. Clin Exp Immunol. 1975 Jan;19(1):17–32. [PMC free article] [PubMed] [Google Scholar]
- Kerman R. H., Kimball P. M., Van Buren C. T., Lewis R. M., DeVera V., Baghdahsarian V., Heydari A., Kahan B. D. Improved renal allograft survival for AHG and DTE/AHG crossmatch-negative recipients. Transplant Proc. 1991 Feb;23(1 Pt 1):400–402. [PubMed] [Google Scholar]
- Lee P., Matsueda G. R., Allen P. M. T cell recognition of fibrinogen. A determinant on the A alpha-chain does not require processing. J Immunol. 1988 Feb 15;140(4):1063–1068. [PubMed] [Google Scholar]
- Lovett A. E., McCarthy M., Wolinsky J. S. Mapping cell-mediated immunodominant domains of the rubella virus structural proteins using recombinant proteins and synthetic peptides. J Gen Virol. 1993 Mar;74(Pt 3):445–452. doi: 10.1099/0022-1317-74-3-445. [DOI] [PubMed] [Google Scholar]
- Marr L. D., Sanchez A., Frey T. K. Efficient in vitro translation and processing of the rubella virus structural proteins in the presence of microsomes. Virology. 1991 Jan;180(1):400–405. doi: 10.1016/0042-6822(91)90046-e. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Verdini A. S., Weber P. C., Salemme F. R., Corradin G. Competitor analogs for defined T cell antigens: peptides incorporating a putative binding motif and polyproline or polyglycine spacers. Cell. 1990 Jan 12;60(1):63–72. doi: 10.1016/0092-8674(90)90716-r. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- McCarthy M., Lovett A., Kerman R. H., Overstreet A., Wolinsky J. S. Immunodominant T-cell epitopes of rubella virus structural proteins defined by synthetic peptides. J Virol. 1993 Feb;67(2):673–681. doi: 10.1128/jvi.67.2.673-681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986 Nov 1;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A., Slagle B., Wolinsky J. S. Anti-idiotypic antibodies to rubella virus. Arch Virol. 1989;107(1-2):159–167. doi: 10.1007/BF01313888. [DOI] [PubMed] [Google Scholar]
- Olson G. B., Dent P. B., Rawls W. E., South M. A., Montgomery J. R., Melnick J. L., Good R. A. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med. 1968 Jul 1;128(1):47–68. doi: 10.1084/jem.128.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou D., Chong P., Choi Y., McVeigh P., Jefferies W. A., Koloitis G., Tingle A. J., Gillam S. Identification of T-cell epitopes on E2 protein of rubella virus, as recognized by human T-cell lines and clones. J Virol. 1992 Nov;66(11):6788–6793. doi: 10.1128/jvi.66.11.6788-6793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Imarai M., van Bleek G. M., Joyce S., Nathenson S. G. Vesicular stomatitis virus antigenic octapeptide N52-59 is anchored into the groove of the H-2Kb molecule by the side chains of three amino acids and the main-chain atoms of the amino terminus. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3135–3139. doi: 10.1073/pnas.89.7.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Southern P. J., Oldstone M. B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988 Feb;162(2):321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S., McCarthy M., Allen-Cannady O., Moore W. T., Jin R., Cao S. N., Lovett A., Simmons D. Monoclonal antibody-defined epitope map of expressed rubella virus protein domains. J Virol. 1991 Aug;65(8):3986–3994. doi: 10.1128/jvi.65.8.3986-3994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky J. S., Sukholutsky E., Moore W. T., Lovett A., McCarthy M., Adame B. An antibody- and synthetic peptide-defined rubella virus E1 glycoprotein neutralization domain. J Virol. 1993 Feb;67(2):961–968. doi: 10.1128/jvi.67.2.961-968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]



