Abstract
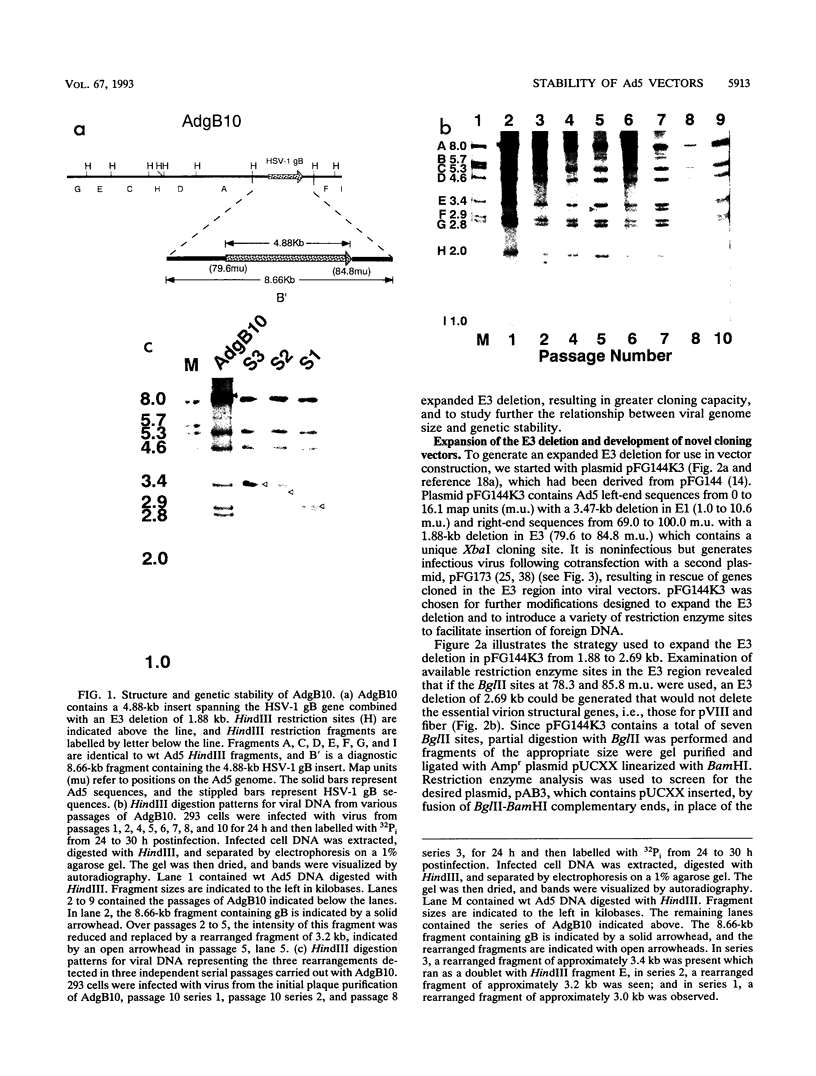
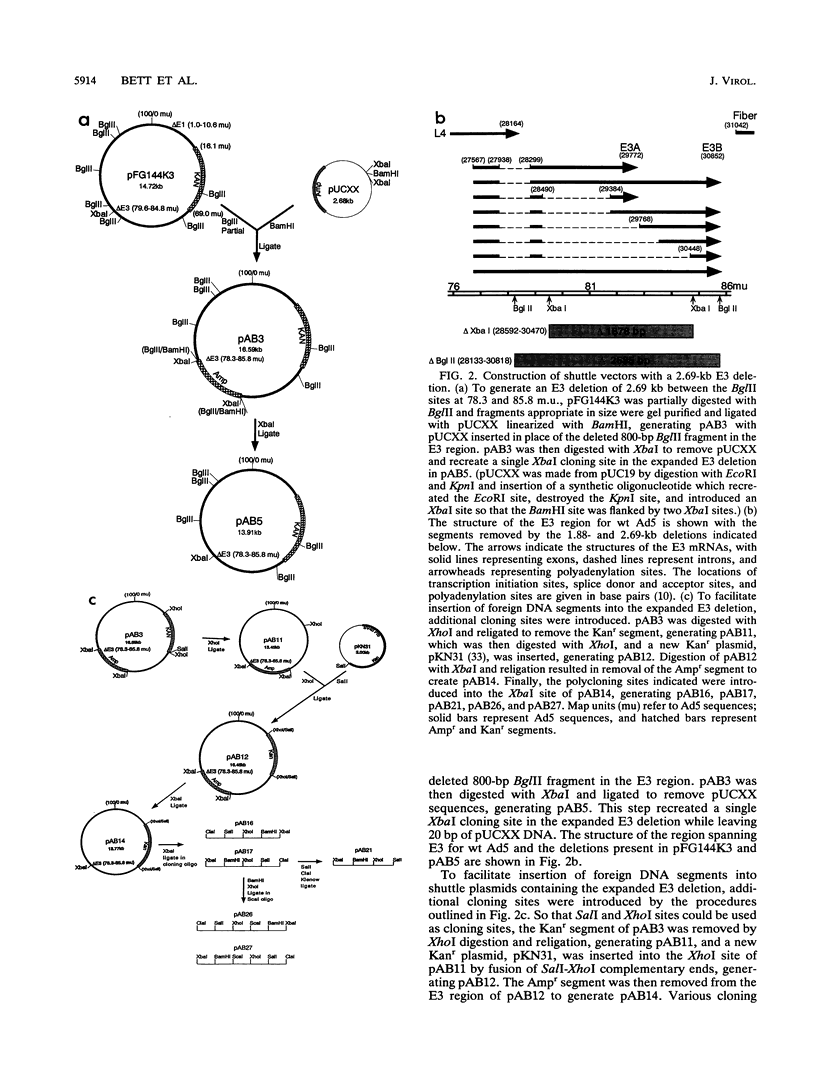
Adenovirus vectors are extensively used for high-level expression of proteins in mammalian cells and are receiving increasing attention for their potential use as live recombinant vaccines and as transducing viruses for use in gene therapy. Although it is commonly argued that one of the chief advantages of adenovirus vectors is their relative stability, this has not been thoroughly investigated. To examine the genetic stability of adenovirus type 5 vectors and in particular to examine the relationship between genetic stability and genome size, adenovirus vectors were constructed with inserts of 4.88 (herpes simplex virus type 1 gB), 4.10 (herpes simplex virus type 1 gB), or 3.82 (LacZ) kb combined with a 1.88-kb E3 deletion or with a newly generated 2.69-kb E3 deletion. The net excess of DNA over the wild-type (wt) genome size ranged from 1.13 to 3.00 kb or 3.1 to 8.3%. Analysis of these vectors during serial passage in tissue culture revealed that when the size exceeded 105% of the wt genome length by approximately 1.2 kb (4.88-kb insert combined with a 1.88-kb deletion), the resulting vector grew very poorly and underwent rapid rearrangement, resulting in loss of the insert after only a few passages. In contrast, vectors with inserts resulting in viral DNA close to or less than a net genome size of 105% of that of the wt grew well and were relatively stable. In general, viruses with genomes only slightly above 105% of that of the wt were unstable and the rapidity with which rearrangement occurred correlated with the size of the insert. These findings suggest that there is a relatively tight constraint on the amount of DNA which can be packaged into virions and that exceeding the limit results in a sharply decreased rate of virus growth. The resultant strong selection for variants which have undergone rearrangement, generating smaller genomes, is manifested as genetic instability of the virus population.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L. Expression of heterologous sequences in adenoviral vectors. Curr Top Microbiol Immunol. 1992;158:39–66. doi: 10.1007/978-3-642-75608-5_3. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Schaffhausen B. S., Roberts T. M., Sharp P. A. Abundant expression of polyomavirus middle T antigen and dihydrofolate reductase in an adenovirus recombinant. J Virol. 1987 Apr;61(4):1213–1220. doi: 10.1128/jvi.61.4.1213-1220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Natuk R. J., Dheer S. K., Lubeck M. D., Bhat B. M., Mason B. B., Greenberg L., Mizutani S., Davis A. R., Hung P. P. Helper independent recombinant adenovirus vectors: expression of HIV env or HBV surface antigen. Int Rev Immunol. 1990;7(1):67–77. doi: 10.3109/08830189009061765. [DOI] [PubMed] [Google Scholar]
- Chang X. B., Wilson J. H. Formation of deletions after initiation of simian virus 40 replication: influence of packaging limit of the capsid. J Virol. 1986 May;58(2):393–401. doi: 10.1128/jvi.58.2.393-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton K. M., Artois M., Prevec L., Campbell J. B., Casey G. A., Wandeler A. I., Armstrong J. Oral rabies vaccination of skunks and foxes with a recombinant human adenovirus vaccine. Arch Virol. 1992;123(1-2):169–179. doi: 10.1007/BF01317147. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Lee S. G., Ronchetti-Blume M., Virk K. P., Mizutani S., Eichberg J. W., Davis A., Hung P. P., Hirsch V. M., Chanock R. M. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J Virol. 1992 Nov;66(11):6721–6727. doi: 10.1128/jvi.66.11.6721-6727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengalvala M., Lubeck M. D., Davis A. R., Mizutani S., Molnar-Kimber K., Morin J., Hung P. P. Evaluation of adenovirus type 4 and type 7 recombinant hepatitis B vaccines in dogs. Vaccine. 1991 Jul;9(7):485–490. doi: 10.1016/0264-410x(91)90033-3. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Dewar R. L., Natarajan V., Vasudevachari M. B., Salzman N. P. Synthesis and processing of human immunodeficiency virus type 1 envelope proteins encoded by a recombinant human adenovirus. J Virol. 1989 Jan;63(1):129–136. doi: 10.1128/jvi.63.1.129-136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloit M., Gilardi-Hebenstreit P., Toma B., Perricaudet M. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J Gen Virol. 1990 Oct;71(Pt 10):2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury G., Haj-Ahmad Y., Brinkley P., Rudy J., Graham F. L. Human adenovirus cloning vectors based on infectious bacterial plasmids. Gene. 1986;50(1-3):161–171. doi: 10.1016/0378-1119(86)90321-5. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury G., Haj-Ahmad Y., Graham F. L. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full length genomes. EMBO J. 1987 Jun;6(6):1733–1739. doi: 10.1002/j.1460-2075.1987.tb02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi P., Courtney M., Pavirani A., Perricaudet M. Expression of human alpha 1-antitrypsin using a recombinant adenovirus vector. FEBS Lett. 1990 Jul 2;267(1):60–62. doi: 10.1016/0014-5793(90)80287-s. [DOI] [PubMed] [Google Scholar]
- Graham F. L. Covalently closed circles of human adenovirus DNA are infectious. EMBO J. 1984 Dec 1;3(12):2917–2922. doi: 10.1002/j.1460-2075.1984.tb02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Prevec L. Adenovirus-based expression vectors and recombinant vaccines. Biotechnology. 1992;20:363–390. doi: 10.1016/b978-0-7506-9265-6.50022-1. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haj-Ahmad Y., Graham F. L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J Virol. 1986 Jan;57(1):267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Lulitanond V., Johnson D. C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990 Aug;177(2):437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- Jacobs S. C., Stephenson J. R., Wilkinson G. W. High-level expression of the tick-borne encephalitis virus NS1 protein by using an adenovirus-based vector: protection elicited in a murine model. J Virol. 1992 Apr;66(4):2086–2095. doi: 10.1128/jvi.66.4.2086-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Johnson D. C. Adenovirus vectors as potential vaccines against herpes simplex virus. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S912–S916. doi: 10.1093/clind/13.supplement_11.s912. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Ghosh-Choudhury G., Smiley J. R., Fallis L., Graham F. L. Abundant expression of herpes simplex virus glycoprotein gB using an adenovirus vector. Virology. 1988 May;164(1):1–14. doi: 10.1016/0042-6822(88)90613-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Lamarche N., Massie B., Richer M., Paradis H., Langelier Y. High level expression in 293 cells of the herpes simplex virus type 2 ribonucleotide reductase subunit 2 using an adenovirus vector. J Gen Virol. 1990 Aug;71(Pt 8):1785–1792. doi: 10.1099/0022-1317-71-8-1785. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Davis A. R., Chengalvala M., Natuk R. J., Morin J. E., Molnar-Kimber K., Mason B. B., Bhat B. M., Mizutani S., Hung P. P. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6763–6767. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangeli A., Danel C., Rosenfeld M. A., Stratford-Perricaudet L., Perricaudet M., Pavirani A., Lecocq J. P., Crystal R. G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993 Jan;91(1):225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Mittal S. K., McDermott M. R., Johnson D. C., Prevec L., Graham F. L. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993 Apr;28(1):67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevec L., Campbell J. B., Christie B. S., Belbeck L., Graham F. L. A recombinant human adenovirus vaccine against rabies. J Infect Dis. 1990 Jan;161(1):27–30. doi: 10.1093/infdis/161.1.27. [DOI] [PubMed] [Google Scholar]
- Prevec L., Christie B. S., Laurie K. E., Bailey M. M., Graham F. L., Rosenthal K. L. Immune response to HIV-1 gag antigens induced by recombinant adenovirus vectors in mice and rhesus macaque monkeys. J Acquir Immune Defic Syndr. 1991;4(6):568–576. [PubMed] [Google Scholar]
- Quantin B., Perricaudet L. D., Tajbakhsh S., Mandel J. L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Ranheim T. S., Shisler J., Horton T. M., Wold L. J., Gooding L. R., Wold W. S. Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J Virol. 1993 Apr;67(4):2159–2167. doi: 10.1128/jvi.67.4.2159-2167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Schneider M., Graham F. L., Prevec L. Expression of the glycoprotein of vesicular stomatitis virus by infectious adenovirus vectors. J Gen Virol. 1989 Feb;70(Pt 2):417–427. doi: 10.1099/0022-1317-70-2-417. [DOI] [PubMed] [Google Scholar]
- Shenk T., Williams J. Genetic analysis of adenoviruses. Curr Top Microbiol Immunol. 1984;111:1–39. doi: 10.1007/978-3-642-69549-0_1. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Hager G. L., Pike J. W., Marx S. J. Overexpression of the human vitamin D3 receptor in mammalian cells using recombinant adenovirus vectors. Mol Endocrinol. 1991 Jun;5(6):867–878. doi: 10.1210/mend-5-6-867. [DOI] [PubMed] [Google Scholar]
- Spessot R., Inchley K., Hupel T. M., Bacchetti S. Cloning of the herpes simplex virus ICP4 gene in an adenovirus vector: effects on adenovirus gene expression and replication. Virology. 1989 Feb;168(2):378–387. doi: 10.1016/0042-6822(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Wang Q., Konan V., Taylor M. W. Expression of the APRT gene in an adenovirus vector system as a model for studying gene therapy. Adv Exp Med Biol. 1991;309B:61–66. doi: 10.1007/978-1-4615-7703-4_14. [DOI] [PubMed] [Google Scholar]
- Ye W. W., Mason B. B., Chengalvala M., Cheng S. M., Zandle G., Lubeck M. D., Lee S. G., Mizutani S., Davis A. R., Hung P. P. Co-expression of hepatitis B virus antigens by a non-defective adenovirus vaccine vector. Arch Virol. 1991;118(1-2):11–27. doi: 10.1007/BF01311300. [DOI] [PubMed] [Google Scholar]
- Yuasa T., Kajino K., Saito I., Miyamura T. Preferential expression of the large hepatitis B virus surface antigen gene by an adenovirus-hepatitis B virus recombinant. J Gen Virol. 1991 Aug;72(Pt 8):1927–1934. doi: 10.1099/0022-1317-72-8-1927. [DOI] [PubMed] [Google Scholar]






