Abstract
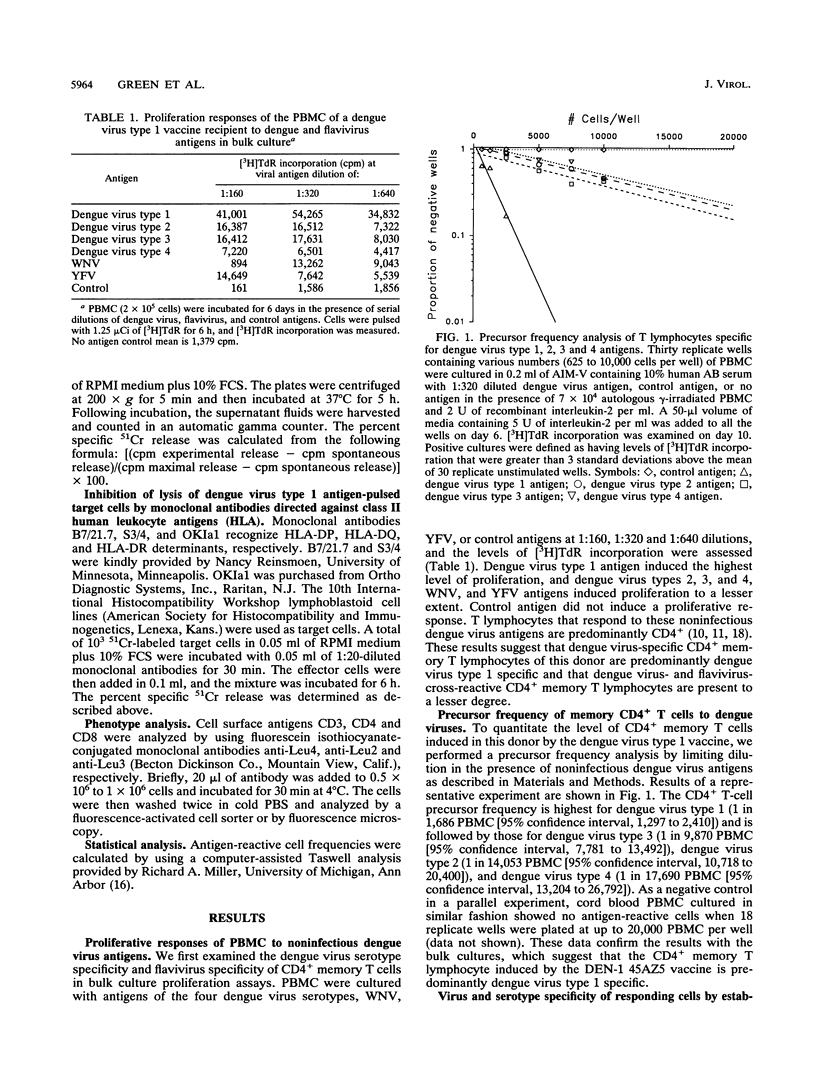
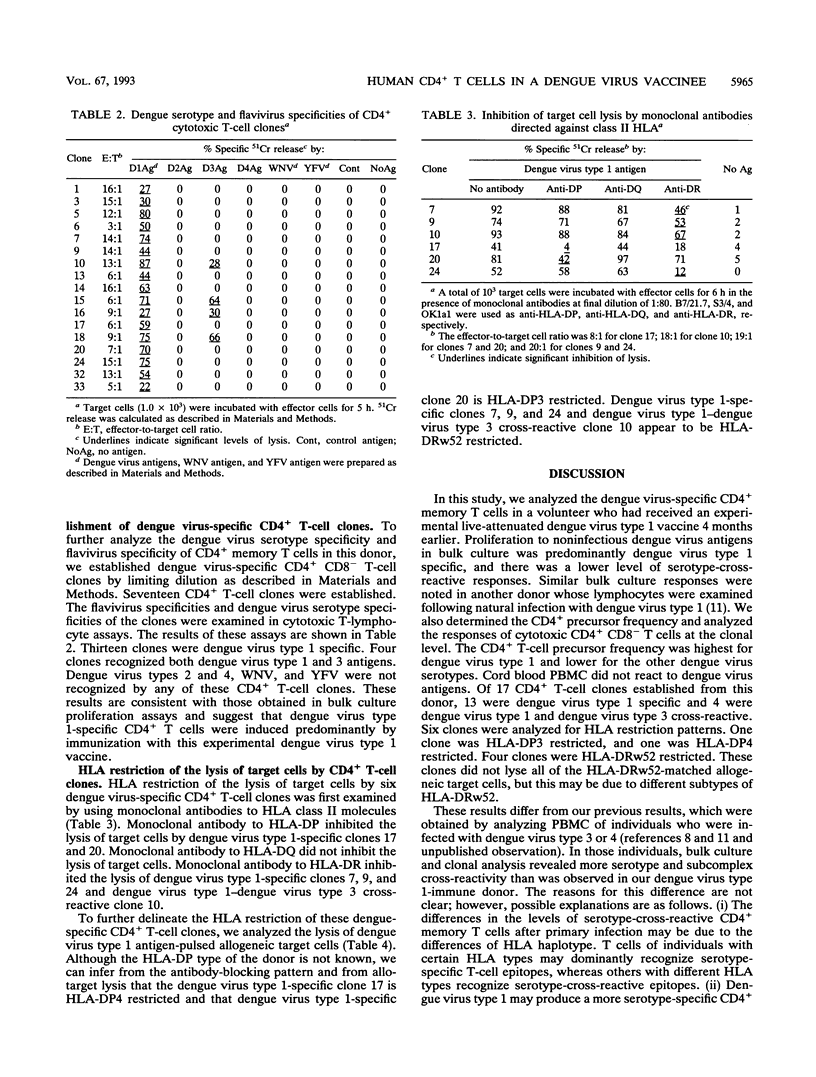
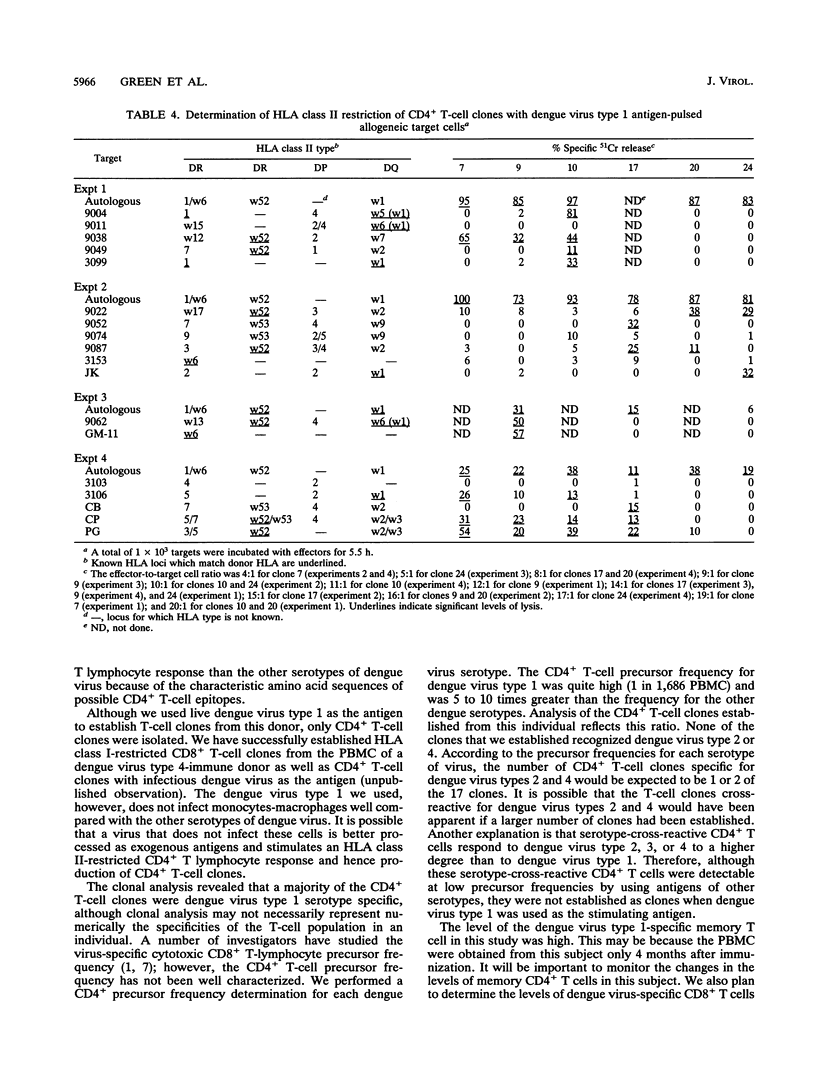
We analyzed the CD4+ T-lymphocyte responses to dengue, West Nile, and yellow fever viruses 4 months after immunization of a volunteer with an experimental live-attenuated dengue virus type 1 vaccine (DEN-1 45AZ5). We examined bulk culture proliferation to noninfectious antigens, determined the precursor frequency of specific CD4+ T cells by limiting dilution, and established and analyzed CD4+ T-cell clones. Bulk culture proliferation was predominantly dengue virus type 1 specific with a lesser degree of cross-reactive responses to other dengue virus serotypes, West Nile virus, and yellow fever virus. Precursor frequency determination by limiting dilution in the presence of noninfectious dengue virus antigens revealed a frequency of antigen-reactive cells of 1 in 1,686 peripheral blood mononuclear cells (PBMC) for dengue virus type 1, 1 in 9,870 PBMC for dengue virus type 3, 1 in 14,053 PBMC for dengue virus type 2, and 1 in 17,690 PBMC for dengue virus type 4. Seventeen CD4+ T-cell clones were then established by using infectious dengue virus type 1 as antigen. Two patterns of dengue virus specificity were found in these clones. Thirteen clones were dengue virus type 1 specific, and four clones recognized both dengue virus types 1 and 3. Analysis of human leukocyte antigen (HLA) restriction revealed that five clones are HLA-DRw52 restricted, one clone is HLA-DP3 restricted, and one clone is HLA-DP4 restricted. These results indicate that in this individual, the CD4+ T-lymphocyte responses to immunization with live-attenuated dengue virus type 1 vaccine are predominantly serotype specific and suggest that a multivalent vaccine may be necessary to elicit strong serotype-cross-reactive CD4+ T-lymphocyte responses in such individuals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borysiewicz L. K., Graham S., Hickling J. K., Mason P. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells: their precursor frequency and stage specificity. Eur J Immunol. 1988 Feb;18(2):269–275. doi: 10.1002/eji.1830180214. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Kingston A. E., Colston M. J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987 Jun;68(3):510–520. [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Kurane I., Lai C. J., Bray M., Falgout B., Ennis F. A. Dengue virus-specific cross-reactive CD8+ human cytotoxic T lymphocytes. J Virol. 1989 Dec;63(12):5086–5091. doi: 10.1128/jvi.63.12.5086-5091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Hayes E. B., Gubler D. J. Dengue and dengue hemorrhagic fever. Pediatr Infect Dis J. 1992 Apr;11(4):311–317. doi: 10.1097/00006454-199204000-00010. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Kurane I., Brinton M. A., Samson A. L., Ennis F. A. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991 Apr;65(4):1823–1828. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Innis B. L., Nisalak A., Hoke C., Nimmannitya S., Meager A., Ennis F. A. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Invest. 1989 Feb;83(2):506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Meager A., Ennis F. A. Dengue virus-specific human T cell clones. Serotype crossreactive proliferation, interferon gamma production, and cytotoxic activity. J Exp Med. 1989 Sep 1;170(3):763–775. doi: 10.1084/jem.170.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee K. T., Jr, Bancroft W. H., Eckels K. H., Redfield R. R., Summers P. L., Russell P. K. Lack of attenuation of a candidate dengue 1 vaccine (45AZ5) in human volunteers. Am J Trop Med Hyg. 1987 Mar;36(2):435–442. doi: 10.4269/ajtmh.1987.36.435. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Research on dengue during World War II. Am J Trop Med Hyg. 1952 Jan;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Sekhon G. S., Kennett R., Bodmer W. F., Bodmer J. Permanent lymphoid lines from genetically marked lymphocytes: success with lymphocytes recovered from frozen storage. Tissue Antigens. 1976 Mar;7(3):165–172. doi: 10.1111/j.1399-0039.1976.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Zivny J., Kurane I., Tacket C. O., Edelman R., Ennis F. A. Dengue virus-specific, human CD4+ cytotoxic T lymphocytes generated in short-term culture. Viral Immunol. 1993 Summer;6(2):143–151. doi: 10.1089/vim.1993.6.143. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]



