Abstract
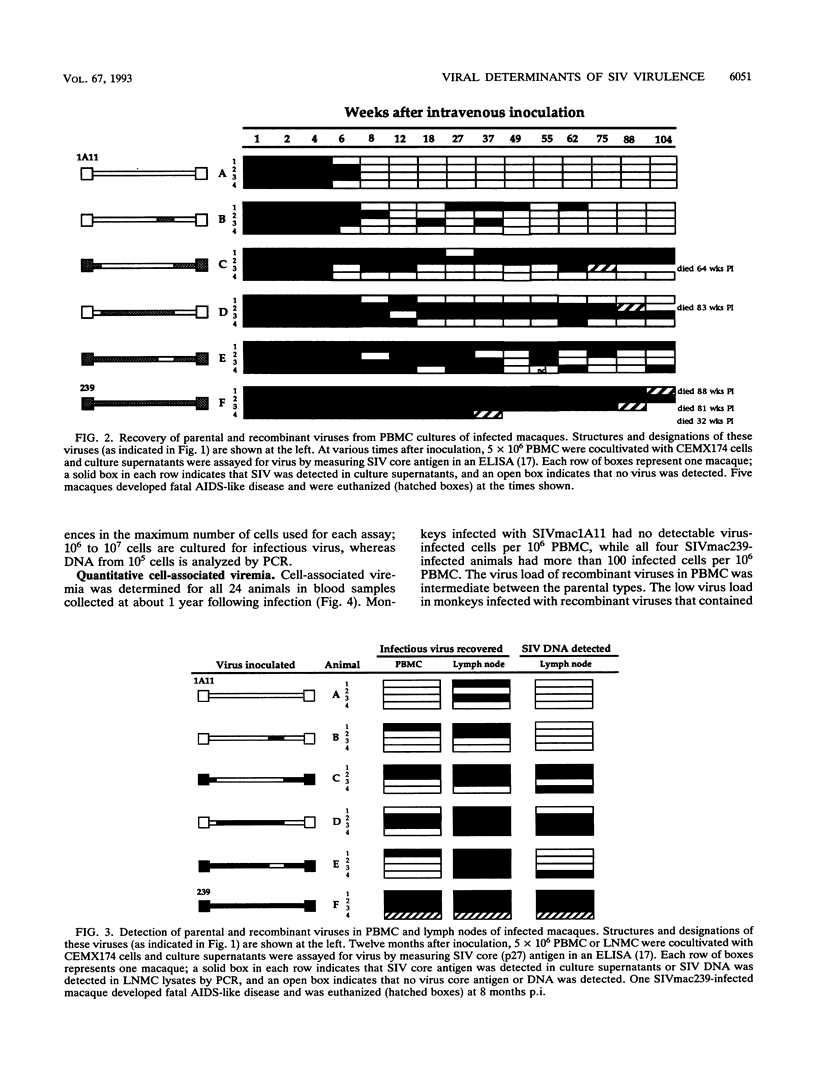
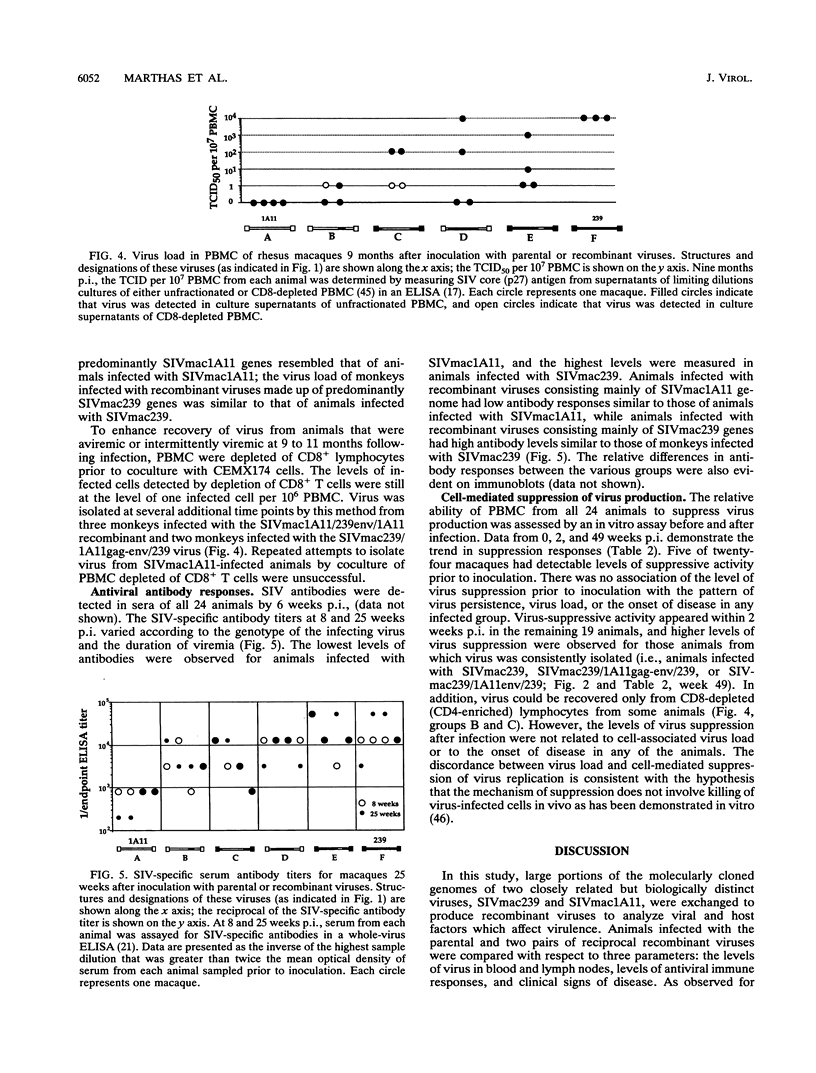
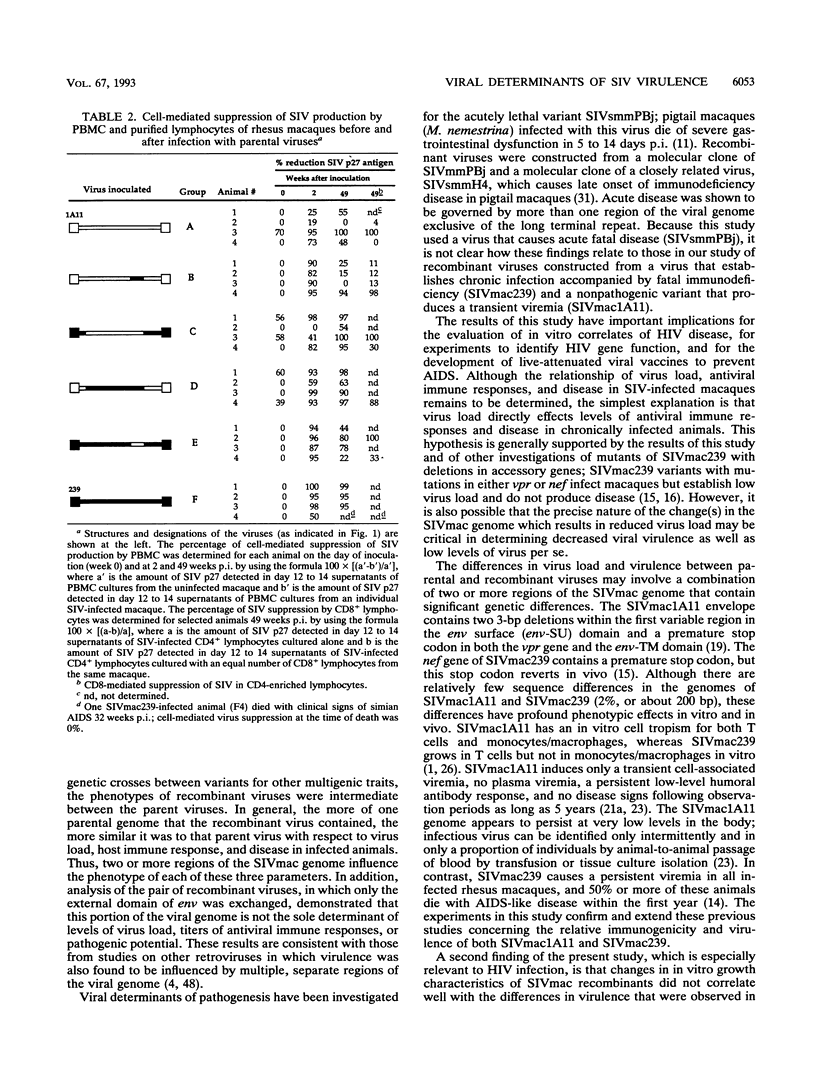
To identify viral determinants of simian immunodeficiency virus (SIV) virulence, two pairs of reciprocal recombinants constructed from a pathogenic (SIVmac239) and a nonpathogenic (SIVmac1A11) molecular clone of SIV were tested in rhesus macaques. A large 6.2-kb fragment containing gag, pol, env, and the regulatory genes from each of the cloned (parental) viruses was exchanged to produce one pair of recombinant viruses (designated SIVmac1A11/239gag-env/1A11 and SIVmac239/1A11gag-env/239 to indicate the genetic origins of the 5'/internal/3' regions, respectively, of the virus). A smaller 1.4-kb fragment containing the external env domain of each of the parental viruses was exchanged to create the second pair (SIVmac1A11/239env/1A11 and SIVmac239/1A11env/239) of recombinant viruses. Each of the two parental and four recombinant viruses was inoculated intravenously into four rhesus macaques, and all 24 animals were viremic by 4 weeks postinoculation (p.i.). Virus could not be isolated from peripheral blood mononuclear cells (PBMC) of any animals infected with SIVmac1A11 after 6 weeks p.i. but was consistently isolated from all macaques inoculated with SIVmac239 for 92 weeks p.i. Virus isolation was variable from animals infected with recombinant viruses; SIVmac1A11/239gag-env/1A11 and SIVmac239/1A11env/239 were isolated most frequently. Animals inoculated with SIVmac239 had 10 to 100 times more virus-infected PBMC than those infected with recombinant viruses. Three animals infected with SIVmac239 died with simian AIDS (SAIDS) during the 2-year observation period after inoculation, and the fourth SIVmac239-infected animal had clinical signs of SAIDS. Two animals infected with recombinant viruses died with SAIDS; one was infected with SIVmac239/1A11gag-env/239, and the other was infected with SIVmac1A11/239gag-env/1A11. The remaining 18 macaques remained healthy by 2 years p.i., and 13 were aviremic. One year after inoculation, peripheral lymph nodes of some of these healthy, aviremic animals harbored infected cells. All animals seroconverted within the first few weeks of infection, and the magnitude of antibody response to SIV was proportional to the levels and duration of viremia. Virus-suppressive PBMC were detected within 2 to 4 weeks p.i. in all animals but tended to decline as viremia disappeared. There was no association of levels of cell-mediated virus-suppressive activity and either virus load or disease progression. Taken together, these results indicate that differences in more than one region of the viral genome are responsible for the lack of virulence of SIVmac1A11.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banapour B., Marthas M. L., Munn R. J., Luciw P. A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). Virology. 1991 Jul;183(1):12–19. doi: 10.1016/0042-6822(91)90113-p. [DOI] [PubMed] [Google Scholar]
- Banapour B., Marthas M. L., Ramos R. A., Lohman B. L., Unger R. E., Gardner M. B., Pedersen N. C., Luciw P. A. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991 Nov;65(11):5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Blais B. P., Robinson H. L. Long terminal repeat (LTR) sequences, env, and a region near the 5' LTR influence the pathogenic potential of recombinants between Rous-associated virus types 0 and 1. J Virol. 1988 Sep;62(9):3431–3437. doi: 10.1128/jvi.62.9.3431-3437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Robinson H. L. Influence of env and long terminal repeat sequences on the tissue tropism of avian leukosis viruses. J Virol. 1988 Dec;62(12):4828–4831. doi: 10.1128/jvi.62.12.4828-4831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Hansen-Moosa A., Mori K., Bouvier D. P., King N. W., Daniel M. D., Ringler D. J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991 Jul;139(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Switzer W. M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res Hum Retroviruses. 1989 Aug;5(4):397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Bonser S. E., Rackowski J. L., Shan H., Kanki P. J. Biological characterization of a simian immunodeficiency virus-like retrovirus (HTLV-IV): evidence for CD4-associated molecules required for infection. J Virol. 1988 Aug;62(8):2557–2568. doi: 10.1128/jvi.62.8.2557-2568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Lang S. M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Müller J., Müller-Hermelink H., Fuchs D., Wachter H., Daniel M. M. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993 Feb;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman B. L., Higgins J., Marthas M. L., Marx P. A., Pedersen N. C. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Oct;29(10):2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Shaw K. E., Unger R. E., Planelles V., Stout M. W., Lackner J. E., Pratt-Lowe E., Leung N. J., Banapour B., Marthas M. L. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res Hum Retroviruses. 1992 Mar;8(3):395–402. doi: 10.1089/aid.1992.8.395. [DOI] [PubMed] [Google Scholar]
- Marthas M. L., Banapour B., Sutjipto S., Siegel M. E., Marx P. A., Gardner M. B., Pedersen N. C., Luciw P. A. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J Med Primatol. 1989;18(3-4):311–319. [PubMed] [Google Scholar]
- Marthas M. L., Miller C. J., Sutjipto S., Higgins J., Torten J., Lohman B. L., Unger R. E., Ramos R. A., Kiyono H., McGhee J. R. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against vaginal challenge with virulent SIV. J Med Primatol. 1992 Feb-May;21(2-3):99–107. [PubMed] [Google Scholar]
- Marthas M. L., Sutjipto S., Higgins J., Lohman B., Torten J., Luciw P. A., Marx P. A., Pedersen N. C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990 Aug;64(8):3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Desrosiers R. C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993 May;67(5):2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Kodama T., Desrosiers R. C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992 Apr;66(4):2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Hoover E. A., Quackenbush S. L., Donahue P. R. Disease progression and viral genome variants in experimental feline leukemia virus-induced immunodeficiency syndrome. J Acquir Immune Defic Syndr. 1991;4(6):547–557. [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre F. J., Johnson P. R., Lewis M. G., Anderson D. C., Klumpp S., McClure H. M., Hirsch V. M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993 May;67(5):2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Dornsife R. E., Mullins J. I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Reinsch S. S., Shank P. R. Sequences near the 5' long terminal repeat of avian leukosis viruses determine the ability to induce osteopetrosis. J Virol. 1986 Jul;59(1):45–49. doi: 10.1128/jvi.59.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Schatz P. J., Jensen L. M., Tsichlis P. N., Coffin J. M., Robinson H. L. Sequences in the gag-pol-5'env region of avian leukosis viruses confer the ability to induce osteopetrosis. Virology. 1985 Aug;145(1):94–104. doi: 10.1016/0042-6822(85)90204-1. [DOI] [PubMed] [Google Scholar]
- Sutjipto S., Pedersen N. C., Miller C. J., Gardner M. B., Hanson C. V., Gettie A., Jennings M., Higgins J., Marx P. A. Inactivated simian immunodeficiency virus vaccine failed to protect rhesus macaques from intravenous or genital mucosal infection but delayed disease in intravenously exposed animals. J Virol. 1990 May;64(5):2290–2297. doi: 10.1128/jvi.64.5.2290-2297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. E., Marthas M. L., Lackner A. A., Pratt-Lowe E., Lohman B. L., Van Rompay K., Luciw P. A. Detection of simian immunodeficiency virus DNA in macrophages from infected rhesus macaques. J Med Primatol. 1992 Feb-May;21(2-3):74–81. [PubMed] [Google Scholar]
- Unger R. E., Marthas M. L., Pratt-Lowe E., Padrid P. A., Luciw P. A. The nef gene of simian immunodeficiency virus SIVmac1A11. J Virol. 1992 Sep;66(9):5432–5442. doi: 10.1128/jvi.66.9.5432-5442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay K. K., Marthas M. L., Ramos R. A., Mandell C. P., McGowan E. K., Joye S. M., Pedersen N. C. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3'-azido-3'-deoxythymidine prevents SIV infection. Antimicrob Agents Chemother. 1992 Nov;36(11):2381–2386. doi: 10.1128/aac.36.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Erickson A. L., Hsueh F. C., Levy J. A. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol. 1991 Nov;65(11):5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Knupp C., Wong P. K. A 1.6-kilobase-pair fragment in the genome of the ts1 mutant of Moloney murine leukemia virus TB that is associated with temperature sensitivity, nonprocessing of Pr80env, and paralytogenesis. J Virol. 1985 May;54(2):364–373. doi: 10.1128/jvi.54.2.364-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Tzeng E., Knupp C., Wong P. K. The neurovirulent determinants of ts1, a paralytogenic mutant of Moloney murine leukemia virus TB, are localized in at least two functionally distinct regions of the genome. J Virol. 1986 Jul;59(1):59–65. doi: 10.1128/jvi.59.1.59-65.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



