Abstract
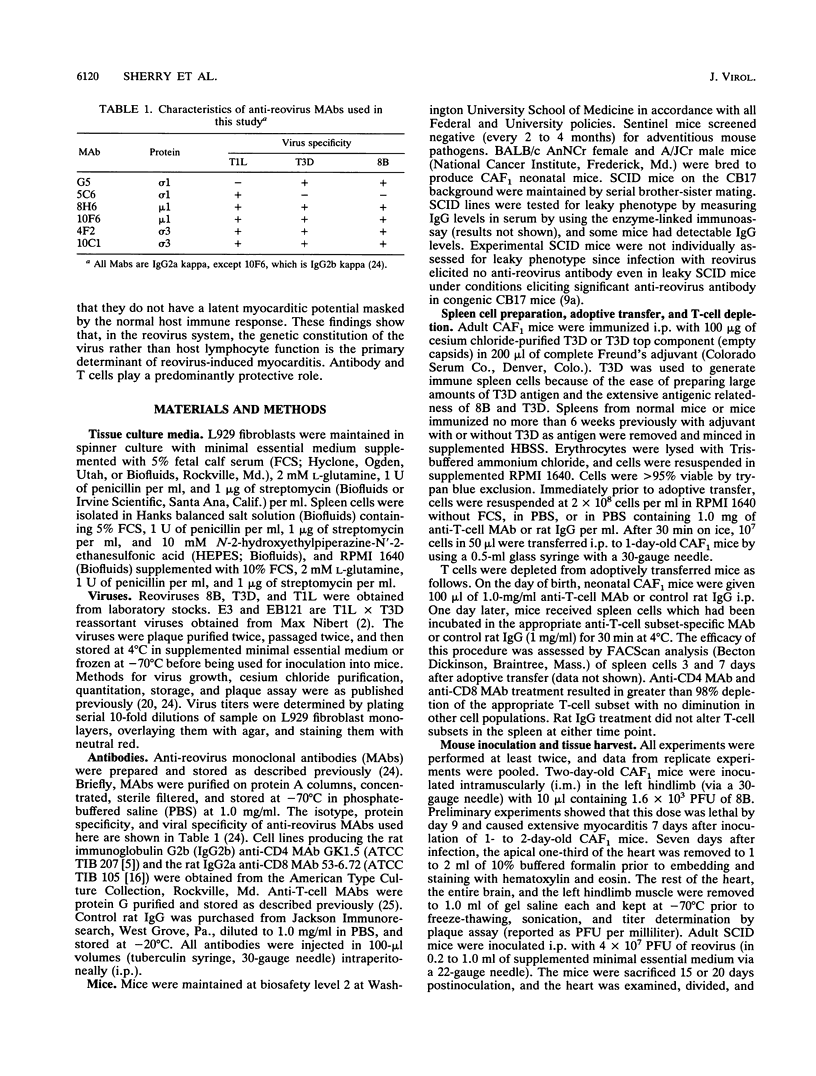
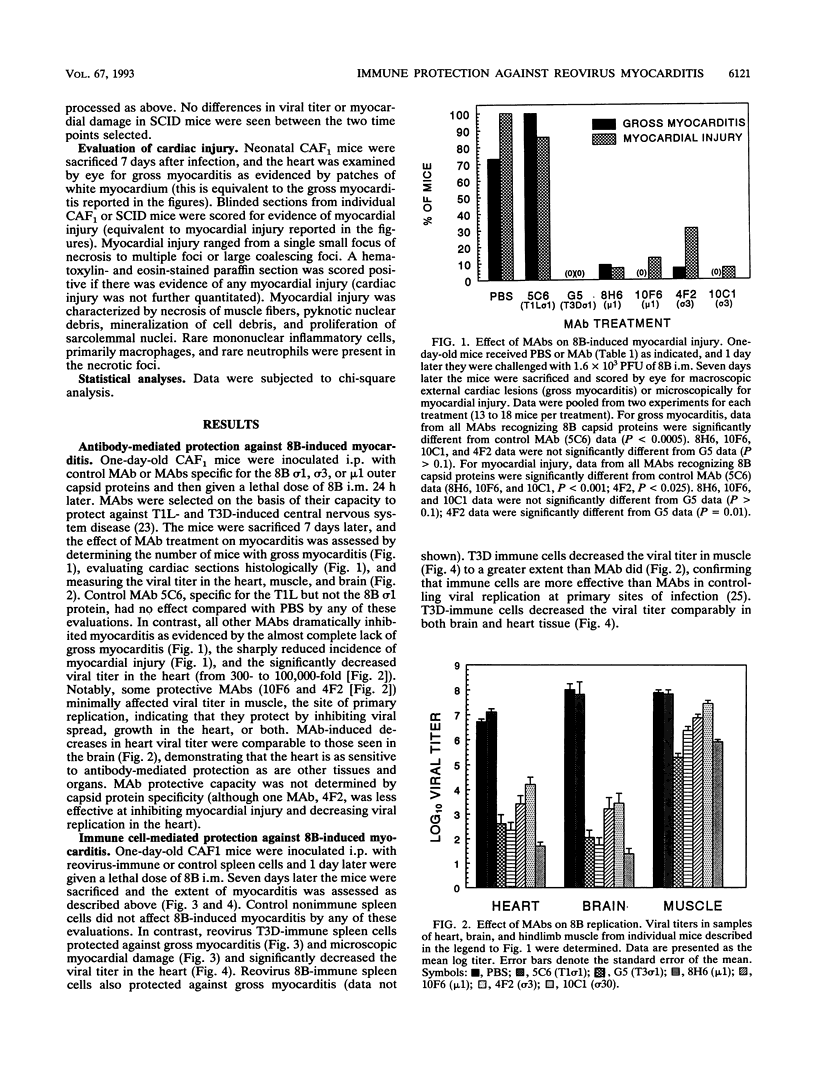
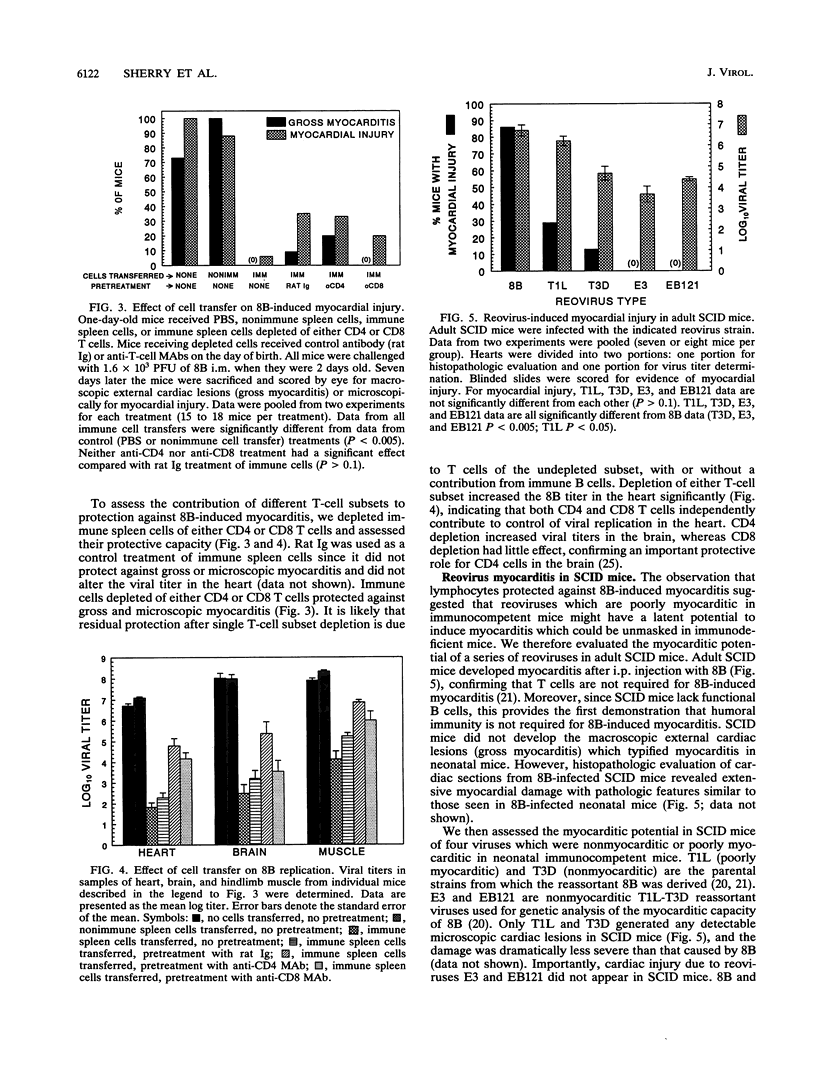
Many studies suggest that host lymphocytes are damaging, rather than protective, in virally induced myocarditis. We have investigated the role of lymphocyte-based immunity in murine myocarditis by using a myocarditic reovirus (reovirus serotype 3 8B), nonmyocarditic reoviruses, adoptive transfer experiments, and mice with severe combined immunodeficiency (SCID mice). Prior to infection, passive transfer of monoclonal antibodies specific for 8B capsid proteins protected neonatal mice against 8B-induced myocarditis, indicating that humoral immunity can protect against myocarditis. Some monoclonal antibodies acted by blocking viral spread to and/or replication in the heart. Passive transfer of reovirus-immune, but not naive, spleen cells prior to infection protected neonatal mice from 8B-induced myocarditis. Depletion of either CD4 or CD8 T cells resulted in increased viral titer in the heart but did not abrogate immune cell-mediated protection against myocardial injury. This shows that both CD4 and CD8 T cells can act independently to protect myocardial tissue from reovirus infection. In addition, reovirus 8B caused extensive myocarditis in SCID mice. This confirms a prior report (B. Sherry, F. J. Schoen, E. Wenske, and B. N. Fields, J. Virol. 63:4840-4849, 1989) that T cells are not required for reovirus-induced myocarditis and demonstrates for the first time that B cells are not required for reovirus-induced myocarditis. We used SCID mice and a panel of reoviruses to assess (i) the relationship between growth in the heart and myocardial damage and (ii) the possibility that nonmyocarditic reoviruses exhibit a myocarditic phenotype in the absence of functional lymphocytes. Growth in the heart was not the sole determinant of myocarditic potential in SCID mice. Although 8B induced myocarditis in SCID mice, no or minimal myocarditis was found in SCID mice infected with four reovirus strains previously shown (B. Sherry and B. N. Fields, J. Virol. 63:4850-4856, 1989) to be nonmyocarditic or poorly myocarditic in normal neonatal mice. We conclude that (i) humoral immunity and cellular immunity are protective against, and not required for, reovirus-induced myocarditis and (ii) the potential to induce cardiac damage is a property of the virus independent of lymphocyte-based immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty C. J., Sherry B. Cytopathogenic effect in cardiac myocytes but not in cardiac fibroblasts is correlated with reovirus-induced acute myocarditis. J Virol. 1993 Oct;67(10):6295–6298. doi: 10.1128/jvi.67.10.6295-6298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. H., Beisel K. W., McManus B. M. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992 Jan;66(1):24–31. [PubMed] [Google Scholar]
- Craighead J. E., Martin W. B., Huber S. A. Role of CD4+ (helper) T cells in the pathogenesis of murine cytomegalovirus myocarditis. Lab Invest. 1992 Jun;66(6):755–761. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fohlman J., Ilbäck N. G., Friman G., Morein B. Vaccination of Balb/c mice against enteroviral mediated myocarditis. Vaccine. 1990 Aug;8(4):381–384. doi: 10.1016/0264-410x(90)90098-7. [DOI] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. 1986 Sep 1;137(5):1695–1702. [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Murine natural killer cells limit coxsackievirus B3 replication. J Immunol. 1987 Aug 1;139(3):913–918. [PubMed] [Google Scholar]
- Grun J. B., Schultz M., Finkelstein S. D., Crowell R. L., Landau B. J. Pathogenesis of acute myocardial necrosis in inbred mice infected with coxsackievirus B3. Microb Pathog. 1988 Jun;4(6):417–430. doi: 10.1016/0882-4010(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Heintz N., Tracy R. Coxsackievirus B-3-induced myocarditis. Virus and actinomycin D treatment of myocytes induces novel antigens recognized by cytolytic T lymphocytes. J Immunol. 1988 Nov 1;141(9):3214–3219. [PubMed] [Google Scholar]
- Kishimoto C., Abelmann W. H. In vivo significance of T cells in the development of Coxsackievirus B3 myocarditis in mice. Immature but antigen-specific T cells aggravate cardiac injury. Circ Res. 1990 Sep;67(3):589–598. doi: 10.1161/01.res.67.3.589. [DOI] [PubMed] [Google Scholar]
- Klingel K., Hohenadl C., Canu A., Albrecht M., Seemann M., Mall G., Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyu B., Matsumori A., Sato Y., Okada I., Chapman N. M., Tracy S. Cardiac persistence of cardioviral RNA detected by polymerase chain reaction in a murine model of dilated cardiomyopathy. Circulation. 1992 Aug;86(2):522–530. doi: 10.1161/01.cir.86.2.522. [DOI] [PubMed] [Google Scholar]
- Lane J. R., Neumann D. A., Lafond-Walker A., Herskowitz A., Rose N. R. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10.A mice. J Exp Med. 1992 Apr 1;175(4):1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H. L., Reed W. D. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992 Mar;75(3):513–519. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A., Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988 Apr;9(4):117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- Sherry B., Fields B. N. The reovirus M1 gene, encoding a viral core protein, is associated with the myocarditic phenotype of a reovirus variant. J Virol. 1989 Nov;63(11):4850–4856. doi: 10.1128/jvi.63.11.4850-4856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Schoen F. J., Wenske E., Fields B. N. Derivation and characterization of an efficiently myocarditic reovirus variant. J Virol. 1989 Nov;63(11):4840–4849. doi: 10.1128/jvi.63.11.4840-4849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K. L., Mann M. A., Fields B. N., Virgin H. W., 4th Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J Virol. 1993 Jun;67(6):3446–3453. doi: 10.1128/jvi.67.6.3446-3453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Mann M. A., Fields B. N., Tyler K. L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991 Dec;65(12):6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Tyler K. L. Role of immune cells in protection against and control of reovirus infection in neonatal mice. J Virol. 1991 Oct;65(10):5157–5164. doi: 10.1128/jvi.65.10.5157-5164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS M. N., LEAK P. J., JOSKE R. A., STANLEY N. F., PERRET D. H. MURINE INFECTION WITH REOVIRUS. 3. PATHOLOGY OF INFECTION WITH TYPES 1 AND 2. Br J Exp Pathol. 1965 Apr;46:200–212. [PMC free article] [PubMed] [Google Scholar]
- Weller A. H., Hall M., Huber S. A. Polyclonal immunoglobulin therapy protects against cardiac damage in experimental coxsackievirus-induced myocarditis. Eur Heart J. 1992 Jan;13(1):115–119. doi: 10.1093/oxfordjournals.eurheartj.a060030. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Brubaker J. O., Vargas-Cortes M., O'Donnell C. L. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991 May 1;173(5):1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener J. R., Bartlett J. A., Joklik W. K. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein mu 2 and the major nonstructural protein mu NS, respectively. Virology. 1989 Apr;169(2):293–304. doi: 10.1016/0042-6822(89)90154-2. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Rose N. R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985 May 1;161(5):1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S., Brown E. G. Nucleotide sequence comparison of the M1 genome segment of reovirus type 1 Lang and type 3 Dearing. Virus Res. 1992 Feb;22(2):159–164. doi: 10.1016/0168-1702(92)90042-8. [DOI] [PubMed] [Google Scholar]



