Abstract
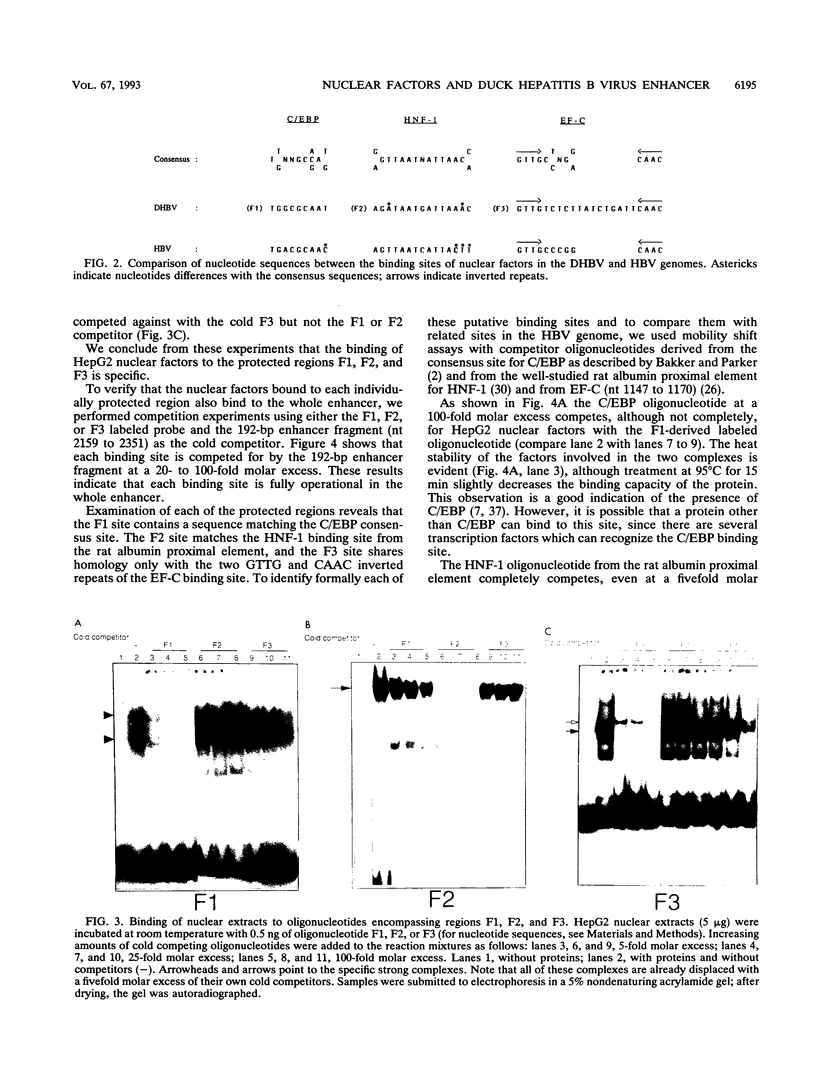
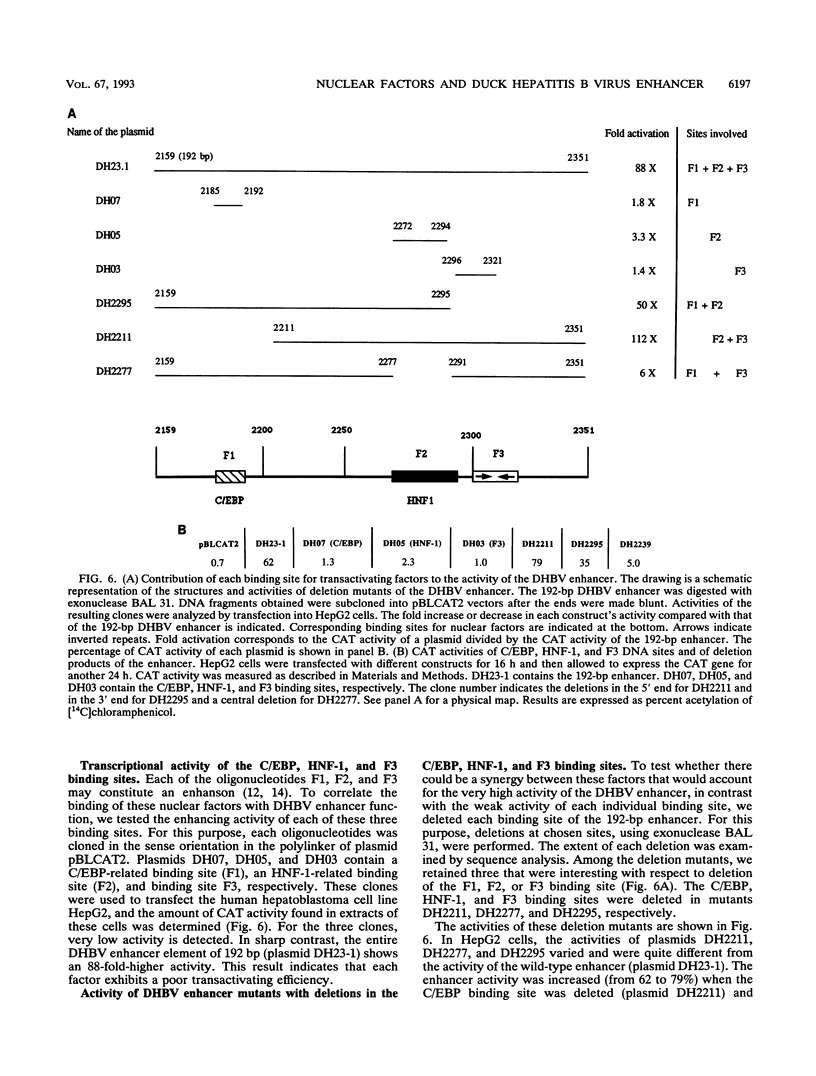
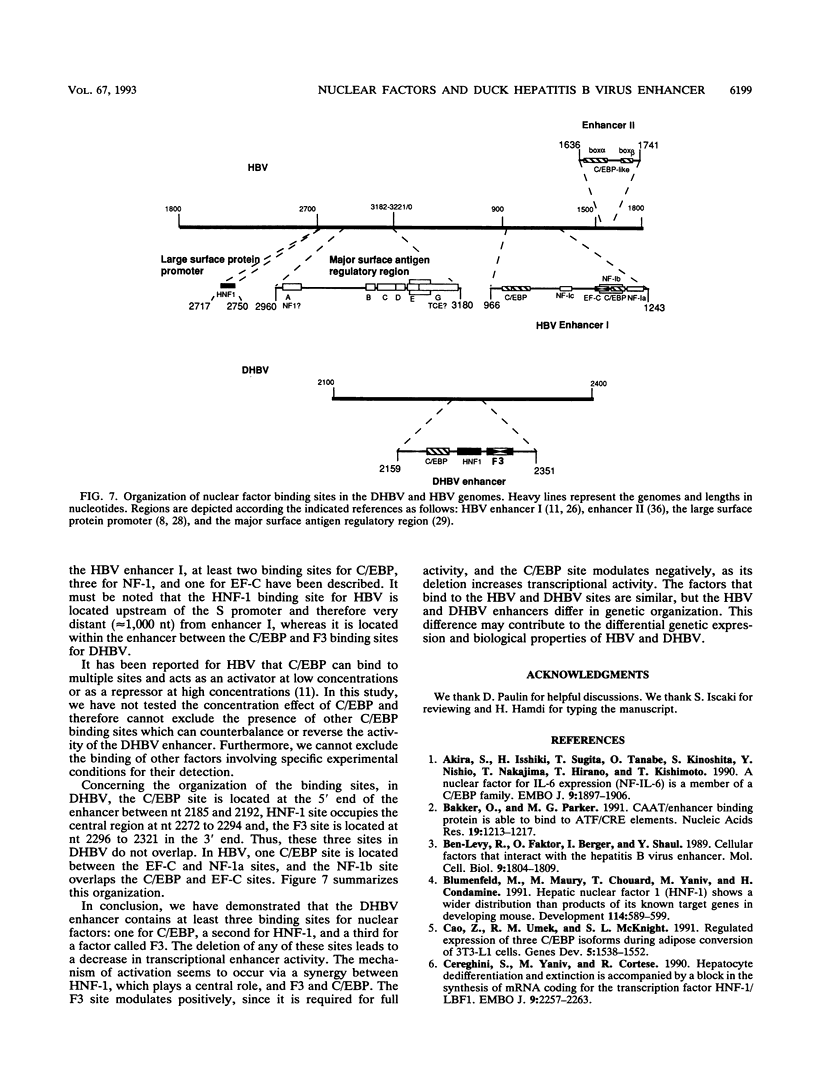
We have analyzed the structures, relative organization, and activities of binding sites for nuclear factors in the duck hepatitis B virus (duck HBV) enhancer. DNase I footprinting analysis and mobility shift assays demonstrate that this enhancer of 192 bp contains at least three binding sites for transcription factors: one for hepatocyte-adipocyte C/EBP, a second for the liver-specific transactivator hepatocyte nuclear factor 1 HNF-1, and a third for a factor, called F3, which binds to a DNA sequence bearing some resemblance to that for the ubiquitous factor EF-C. Analysis of transcriptional activity reveals that oligonucleotides corresponding to the individual binding sites, inserted upstream from a heterologous promoter, display very weak enhancer activity, whereas the enhancer encompassing these three sites displays very high activity. Analysis of duck HBV enhancer mutants indicates that the deletion of any of these sites leads to a modification of transcriptional enhancer activity. The hepatocyte nuclear factor 1 binding site is crucial, since an internal deletion of 14 bp abolishes the activity. The C/EBP site can act as repressor, and the F3 site is required for full activity. Comparative analysis reveals that the nuclear factors are similar to those bound to the human HBV enhancer but that the organization of their binding sites in the duck HBV enhancer is different.
Full text
PDF


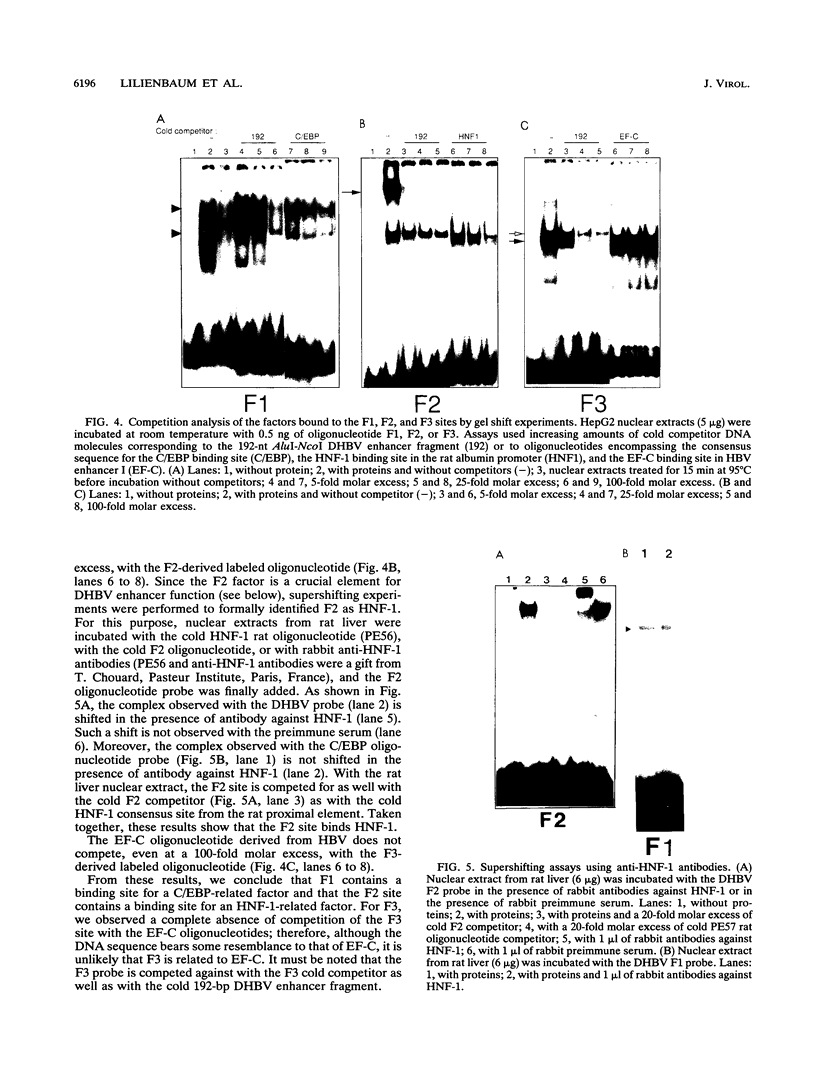


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker O., Parker M. G. CAAT/enhancer binding protein is able to bind to ATF/CRE elements. Nucleic Acids Res. 1991 Mar 25;19(6):1213–1217. doi: 10.1093/nar/19.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Levy R., Faktor O., Berger I., Shaul Y. Cellular factors that interact with the hepatitis B virus enhancer. Mol Cell Biol. 1989 Apr;9(4):1804–1809. doi: 10.1128/mcb.9.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld M., Maury M., Chouard T., Yaniv M., Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. 1991 Oct;113(2):589–599. doi: 10.1242/dev.113.2.589. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Yaniv M., Cortese R. Hepatocyte dedifferentiation and extinction is accompanied by a block in the synthesis of mRNA coding for the transcription factor HNF1/LFB1. EMBO J. 1990 Jul;9(7):2257–2263. doi: 10.1002/j.1460-2075.1990.tb07396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. K., Wang B. Y., Yuh C. H., Wei C. L., Ting L. P. A liver-specific nuclear factor interacts with the promoter region of the large surface protein gene of human hepatitis B virus. Mol Cell Biol. 1989 Nov;9(11):5189–5197. doi: 10.1128/mcb.9.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo-Chaigne B., Pillot J., Lilienbaum A., Levrero M., Elfassi E. Identification of a strong enhancer element upstream from the pregenomic RNA start site of the duck hepatitis B virus genome. J Virol. 1991 Jul;65(7):3882–3886. doi: 10.1128/jvi.65.7.3882-3886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein R., Faktor O., Shaul Y. Hierarchic and cooperative binding of the rat liver nuclear protein C/EBP at the hepatitis B virus enhancer. Mol Cell Biol. 1990 Aug;10(8):4427–4430. doi: 10.1128/mcb.10.8.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Elfassi E. Broad specificity of the hepatitis B enhancer function. Virology. 1987 Sep;160(1):259–262. doi: 10.1016/0042-6822(87)90069-9. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Kim P. S. Modular structure of transcription factors: implications for gene regulation. Cell. 1991 May 31;65(5):717–719. doi: 10.1016/0092-8674(91)90378-c. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Flores L., Egan J., Newbold J., Mason W. S. Individual cells in tissues of DHBV-infected ducks express antigens crossreactive with those on virus surface antigen particles and immature viral cores. Virology. 1984 Sep;137(2):408–413. doi: 10.1016/0042-6822(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Levrero M., Balsano C., Natoli G., Avantaggiati M. L., Elfassi E. Hepatitis B virus X protein transactivates the long terminal repeats of human immunodeficiency virus types 1 and 2. J Virol. 1990 Jun;64(6):3082–3086. doi: 10.1128/jvi.64.6.3082-3086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Condreay L. D., Burch J. B., Mason W. Characterization of the core promoter and enhancer of duck hepatitis B virus. Virology. 1991 Sep;184(1):242–252. doi: 10.1016/0042-6822(91)90841-x. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Scheirle G., Hearing P. Binding of nuclear factor EF-C to a functional domain of the hepatitis B virus enhancer region. Mol Cell Biol. 1989 Jul;9(7):2787–2797. doi: 10.1128/mcb.9.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D. Q., Shih C. H. Transcriptional activation and repression by cellular DNA-binding protein C/EBP. J Virol. 1990 Apr;64(4):1517–1522. doi: 10.1128/jvi.64.4.1517-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney A. K., Milich D. R., Easton A. J., McLachlan A. Differentiation-specific transcriptional regulation of the hepatitis B virus large surface antigen gene in human hepatoma cell lines. J Virol. 1990 May;64(5):2360–2368. doi: 10.1128/jvi.64.5.2360-2368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney A. K., Milich D. R., McLachlan A. Complex regulation of transcription from the hepatitis B virus major surface antigen promoter in human hepatoma cell lines. J Virol. 1991 Sep;65(9):4805–4811. doi: 10.1128/jvi.65.9.4805-4811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Campos J., Chouard T., Yaniv M., Cereghini S. vHNF1 is a homeoprotein that activates transcription and forms heterodimers with HNF1. EMBO J. 1991 Jun;10(6):1445–1457. doi: 10.1002/j.1460-2075.1991.tb07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Will H. Regulatory sequences of duck hepatitis B virus C gene transcription. J Virol. 1991 Nov;65(11):5693–5701. doi: 10.1128/jvi.65.11.5693-5701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Levinson A. D. Properties of the human hepatitis B virus enhancer: position effects and cell-type nonspecificity. J Virol. 1988 Apr;62(4):1305–1313. doi: 10.1128/jvi.62.4.1305-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen P., Wu X., Sun A. L., Wang H., Zhu Y. A., Li Z. P. A new enhancer element, ENII, identified in the X gene of hepatitis B virus. J Virol. 1990 Aug;64(8):3977–3981. doi: 10.1128/jvi.64.8.3977-3981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- Yuh C. H., Ting L. P. C/EBP-like proteins binding to the functional box-alpha and box-beta of the second enhancer of hepatitis B virus. Mol Cell Biol. 1991 Oct;11(10):5044–5052. doi: 10.1128/mcb.11.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]