Abstract
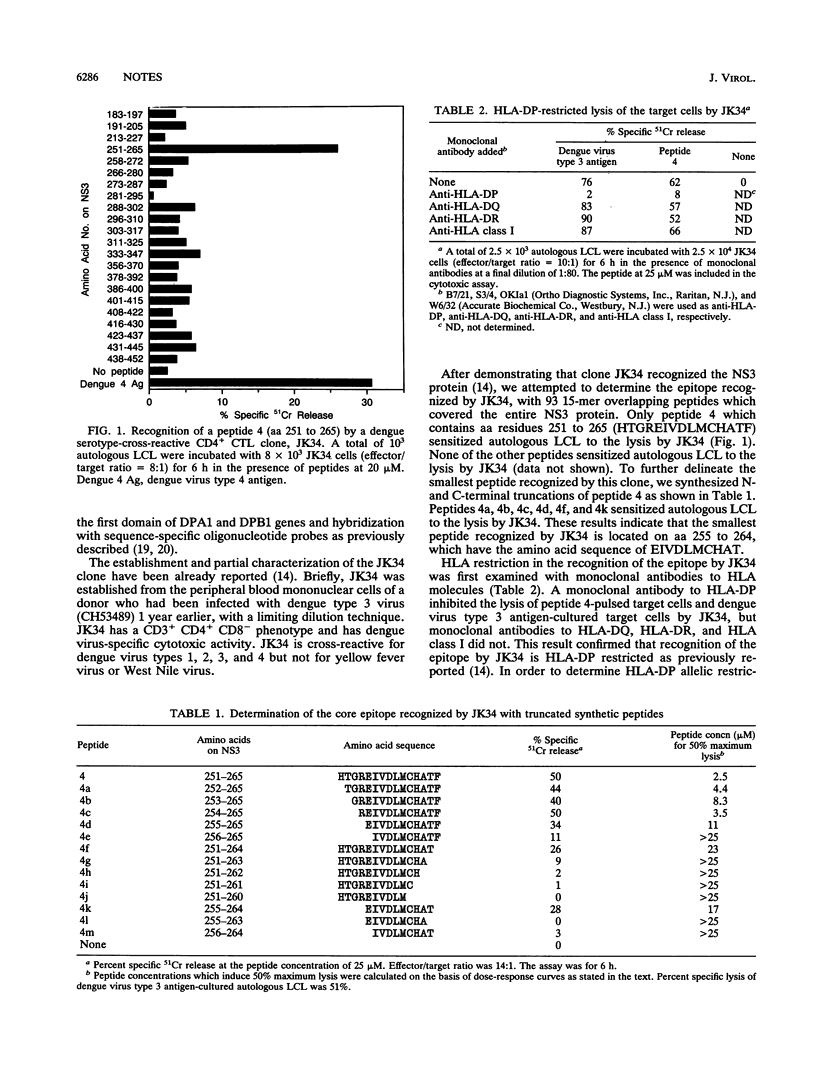
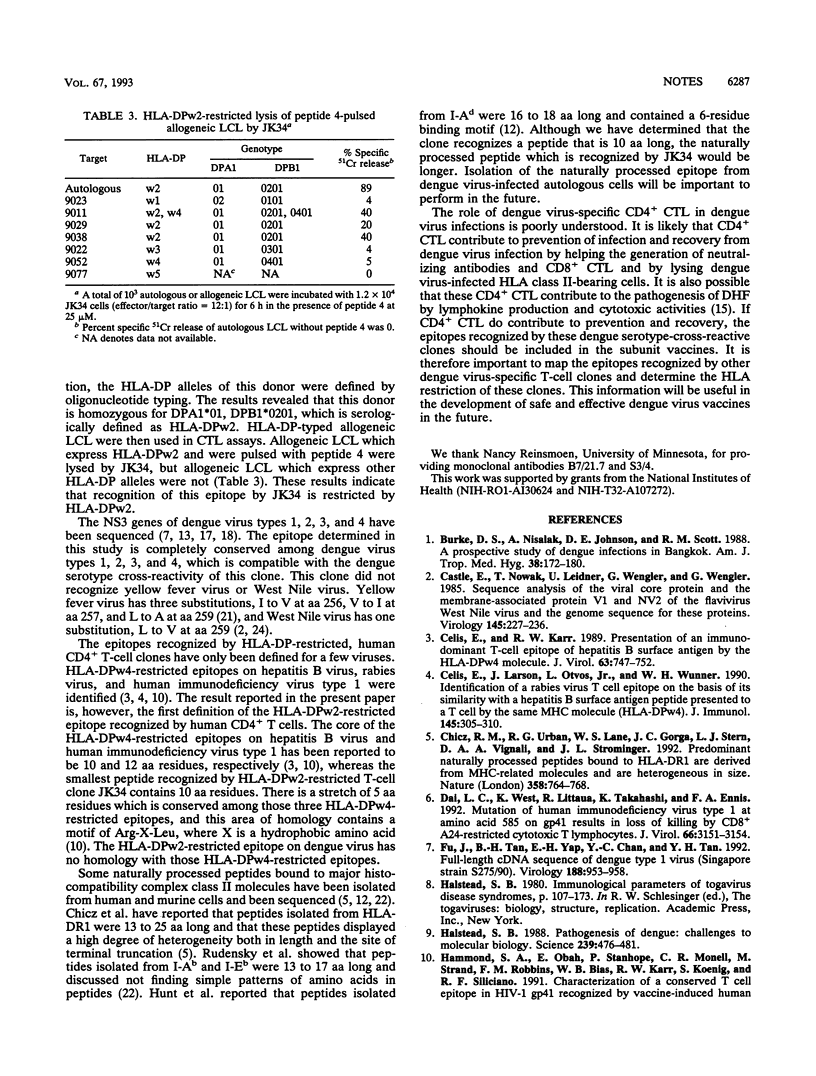
We previously reported that the clone JK34 was cross-reactive for dengue virus types 1, 2, 3, and 4 and recognized NS3 (I. Kurane, M. A. Brinton, A. L. Samson, and F. A. Ennis, J. Virol. 65:1823-1828, 1991). In the present experiments, we defined the epitope at the amino acid level, with 93 15-mer overlapping peptides which cover the entire NS3. A peptide 4 which contains amino acids 251 to 265 of NS3 sensitized the autologous B lymphoblastoid cell line (LCL) to the lysis by JK34. The smallest peptide recognized by JK34 was a 10-mer peptide which contains amino acids 255 to 264 (EIVDLMCHAT). A monoclonal antibody to HLA-DP inhibited the lysis of epitope peptide-pulsed autologous LCL by JK34. Genotypic typing revealed that the HLA-DP of this donor is DPA1*01, DPB1*0201, which is serologically defined as HLA-DPw2. JK34 lysed peptide 4-pulsed allogeneic LCL which carried HLA-DPw2. These results indicate that HLA-DPw2 is the restriction allele for recognition of this epitope by JK34.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke D. S., Nisalak A., Johnson D. E., Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988 Jan;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Castle E., Nowak T., Leidner U., Wengler G., Wengler G. Sequence analysis of the viral core protein and the membrane-associated proteins V1 and NV2 of the flavivirus West Nile virus and of the genome sequence for these proteins. Virology. 1985 Sep;145(2):227–236. doi: 10.1016/0042-6822(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Celis E., Karr R. W. Presentation of an immunodominant T-cell epitope of hepatitis B surface antigen by the HLA-DPw4 molecule. J Virol. 1989 Feb;63(2):747–752. doi: 10.1128/jvi.63.2.747-752.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Larson J., Otvos L., Jr, Wunner W. H. Identification of a rabies virus T cell epitope on the basis of its similarity with a hepatitis B surface antigen peptide presented to T cells by the same MHC molecule (HLA-DPw4). J Immunol. 1990 Jul 1;145(1):305–310. [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Dai L. C., West K., Littaua R., Takahashi K., Ennis F. A. Mutation of human immunodeficiency virus type 1 at amino acid 585 on gp41 results in loss of killing by CD8+ A24-restricted cytotoxic T lymphocytes. J Virol. 1992 May;66(5):3151–3154. doi: 10.1128/jvi.66.5.3151-3154.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Tan B. H., Yap E. H., Chan Y. C., Tan Y. H. Full-length cDNA sequence of dengue type 1 virus (Singapore strain S275/90). Virology. 1992 Jun;188(2):953–958. doi: 10.1016/0042-6822(92)90560-c. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Hammond S. A., Obah E., Stanhope P., Monell C. R., Strand M., Robbins F. M., Bias W. B., Karr R. W., Koenig S., Siliciano R. F. Characterization of a conserved T cell epitope in HIV-1 gp41 recognized by vaccine-induced human cytolytic T cells. J Immunol. 1991 Mar 1;146(5):1470–1477. [PubMed] [Google Scholar]
- Hayes E. B., Gubler D. J. Dengue and dengue hemorrhagic fever. Pediatr Infect Dis J. 1992 Apr;11(4):311–317. doi: 10.1097/00006454-199204000-00010. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Irie K., Mohan P. M., Sasaguri Y., Putnak R., Padmanabhan R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain). Gene. 1989 Feb 20;75(2):197–211. doi: 10.1016/0378-1119(89)90266-7. [DOI] [PubMed] [Google Scholar]
- Kurane I., Brinton M. A., Samson A. L., Ennis F. A. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991 Apr;65(4):1823–1828. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Ennis F. E. Immunity and immunopathology in dengue virus infections. Semin Immunol. 1992 Apr;4(2):121–127. [PubMed] [Google Scholar]
- Kurane I., Innis B. L., Nisalak A., Hoke C., Nimmannitya S., Meager A., Ennis F. A. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Invest. 1989 Feb;83(2):506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E., Makino Y., Zhao B. T., Zhang Y. M., Markoff L., Buckler-White A., Guiler M., Chanock R., Lai C. J. The nucleotide sequence of dengue type 4 virus: analysis of genes coding for nonstructural proteins. Virology. 1987 Aug;159(2):217–228. doi: 10.1016/0042-6822(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Osatomi K., Sumiyoshi H. Complete nucleotide sequence of dengue type 3 virus genome RNA. Virology. 1990 Jun;176(2):643–647. doi: 10.1016/0042-6822(90)90037-r. [DOI] [PubMed] [Google Scholar]
- Reed E., Ho E., Lupu F., McManus P., Vasilescu R., Foca-Rodi A., Suciu-Foca N. Polymorphism of HLA in the Romanian population. Tissue Antigens. 1992 Jan;39(1):8–13. doi: 10.1111/j.1399-0039.1992.tb02148.x. [DOI] [PubMed] [Google Scholar]
- Reed E., Lupu F., McManus P., Seigle R., Suciu-Foca N. Population and family studies of HLA-DR4 by use of oligonucleotide typing. Tissue Antigens. 1992 May;39(5):266–271. doi: 10.1111/j.1399-0039.1992.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Dai L. C., Fuerst T. R., Biddison W. E., Earl P. L., Moss B., Ennis F. A. Specific lysis of human immunodeficiency virus type 1-infected cells by a HLA-A3.1-restricted CD8+ cytotoxic T-lymphocyte clone that recognizes a conserved peptide sequence within the gp41 subunit of the envelope protein. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10277–10281. doi: 10.1073/pnas.88.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Castle E., Leidner U., Nowak T., Wengler G. Sequence analysis of the membrane protein V3 of the flavivirus West Nile virus and of its gene. Virology. 1985 Dec;147(2):264–274. doi: 10.1016/0042-6822(85)90129-1. [DOI] [PubMed] [Google Scholar]


