Abstract
Temperature lability of ADP-glucose pyrophosphorylase (AGP; glucose-1-phosphate adenylyltransferase; ADP: α-d-glucose-1-phosphate adenylyltransferase, EC 2.7.7.27), a key starch biosynthetic enzyme, may play a significant role in the heat-induced loss in maize seed weight and yield. Here we report the isolation and characterization of heat-stable variants of maize endosperm AGP. Escherichia coli cells expressing wild type (WT) Shrunken2 (Sh2), and Brittle2 (Bt2) exhibit a reduced capacity to produce glycogen when grown at 42°C. Mutagenesis of Sh2 and coexpression with WT Bt2 led to the isolation of multiple mutants capable of synthesizing copious amounts of glycogen at this temperature. An increase in AGP stability was found in each of four mutants examined. Initial characterization revealed that the BT2 protein was elevated in two of these mutants. Yeast two-hybrid studies were conducted to determine whether the mutant SH2 proteins more efficiently recruit the BT2 subunit into tetramer assembly. These experiments showed that replacement of WT SH2 with the heat-stable SH2HS33 enhanced interaction between the SH2 and BT2 subunits. In agreement, density gradient centrifugation of heated and nonheated extracts from WT and one of the mutants, Sh2hs33, identified a greater propensity for heterotetramer dissociation in WT AGP. Sequencing of Sh2hs33 and several other mutants identified a His-to-Tyr mutation at amino acid position 333. Hence, a single point mutation in Sh2 can increase the stability of maize endosperm AGP through enhanced subunit interactions.
Temperature stress is a major environmental factor that greatly reduces grain yield and seed weight in many cereal crops such as maize, wheat, and barley. Direct measurements (1–6) as well as historical and climatological studies (7,8) have shown that elevated temperatures decrease the duration of grain filling in maize and decrease seed weight. That the physiological processes of the maturing seed itself are adversely affected by heat stress is evident from studies of in vitro-developed maize kernels. While kernels cultured at 35°C assimilate sugars at normal or elevated rates (9), seed weight is dramatically reduced (10). Similar studies with wheat and barley are consistent with the hypothesis that transport of sucrose to the endosperm is not limiting under high temperature conditions (11–13). Rather, conversion of sugars to storage products is adversely affected.
Because starch accounts for approximately 70% of cereal seed weight, the stressful environmental factors must impact on this biochemical pathway. In this regard, studies from organisms ranging from bacteria to plants point to the important and rate-limiting nature of the starch biosynthetic enzyme, ADP-glucose pyrophosphorylase (AGP; glucose-1-phosphate adenylyltransferase; ADP:α-d-glucose-1-phosphate adenylytransferase, EC 2.7.7.27). Historically, AGP has received considerable attention because of its allosteric properties and its position as the first unique step in starch synthesis (reviewed in refs. 14 and 15). The first definitive evidence for its rate-limiting role in a plant system came with the work of Stark et al. (16). Those investigators increased starch content 30% by expressing an allosterically altered bacterial AGP in the potato tuber. Subsequently, Giroux et al. (17) modified the allosteric properties of the maize endosperm AGP and increased maize seed weight approximately 15%. Hence, AGP represents an important target for genetic manipulation.
Of significance to the heat-induced yield loss is the fact that maize endosperm AGP is heat labile (18, 19). Maize endosperm AGP loses 96% of its activity when heated in vitro at 57°C for 5 min. This result is in sharp contrast to potato AGP that is fully stable at 60°C (20, 21). Singletary et al. (12) extended the studies of the heat lability of maize endosperm AGP by monitoring seed weight and various enzymic activities throughout development in in vitro-developed kernels grown at various temperatures. Seed weight decreased as temperature increased from 22°C to 36°C. Although AGP and soluble starch synthase activities were most negatively impacted by elevated temperature, Singletary et al. (12) concluded that AGP is likely more important in the premature cessation of starch deposition. In agreement, Duke and Doehlert (22) monitored a number of starch synthetic enzymes and their transcripts in kernels developed at elevated temperatures. AGP activity was most sensitive to elevated temperatures. These investigators postulated that AGP might have a faster turnover rate under heat stress conditions compared with other enzymes assayed. Although the maize investigations point to the heat lability of AGP as the major cause of heat-induced weight loss, studies in wheat suggest that loss of soluble starch synthase activity is the cause of heat-induced seed weight loss (23–26).
AGP is composed of two large and two small subunits. The endosperm-specific maize Shrunken2 (Sh2) gene encodes the large subunit, whereas the small subunit is encoded by Brittle2 (Bt2) (27–29). Analysis of maize mutants shows that lack of AGP leads to a reduction in starch content and, in turn, to shrunken, brittle, or collapsed kernels at seed maturity.
In the studies reported here, we address the temperature-sensitive nature of maize endosperm AGP and exploit a bacterial expression system to isolate heat-stable variants. One mutation was found repeatedly. This mutation conditions heat-stable maize endosperm AGP activity by enhancing interactions between the SH2 and BT2 subunits.
MATERIALS AND METHODS
Mutagenesis and Selection.
Plasmid DNA containing the coding region of wild-type (WT) Sh2 cDNA (17) was treated with the mutagen hydoxylamine as previously described (30). Treated Sh2 plasmid was transformed into Escherichia coli AC70R1–504 containing the WT Bt2 coding region on a compatible expression vector (17). AC70R1–504 lacks the endogenous bacterial AGP because of mutation at glgC (31). Bacteria were plated on the medium used by Govons et al. (32) except the glucose concentration was reduced to 0.1%. AC70R1–504 cells expressing WT Sh2 and Bt2 exhibit a reduced capacity to complement the glgC− mutation of AC70R1–504 on this medium when grown at 42°C. Variants with an increased capacity to produce glycogen at 42°C were isolated by their rapid, more intense iodine staining compared with WT Sh2 and Bt2. Coding regions for mutants were cloned into an unmutated vector and confirmed by sequencing.
AGP Enzyme Analysis.
A 2-ml Luria broth culture containing spectinomycin (100 μg/ml) and kanamycin (75 μg/ml) was inoculated from a glycerol stock of AC70R1–504 E. coli cells expressing WT or mutant Sh2 and WT Bt2 and grown overnight at 37°C. This culture was used to inoculate a 100-ml culture of Luria broth (100 μg/ml of spectinomycin and 75 μg/ml of kanamycin). The culture was grown to an OD600 = 1.2 and induced for 12 hr by addition of isopropyl β-d-thiogalactoside and nalidixic acid at final concentrations of 0.2 mM and 25 μg/ml, respectively. Cells were harvested as previously described (33). The cell pellet was resuspended in 50 mM Hepes, pH 7.5, 10 mM KPi, pH 7.5, 5 mM MgCl2, 5 mM EDTA, 20% sucrose, and 30% ammonium sulfate. DTT (1 mM), 50 μg/ml of lysozyme, 1 μg/ml of pepstatin, 1 μg/ml of leupeptin, 10 μg/ml of chymostatin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM benzamidine were added just before use. Lysate was sonicated three times for 10 sec with incubation on ice between steps. Sample was centrifuged for 5 min at 12,500 rpm at 4°C, and the supernatant was stored on ice.
Aliquots (0.5 ml) of crude extract were placed in a 60°C water bath for 6 min with gentle mixing, cooled on ice, and then clarified by centrifugation at 4°C. Bradford assays were used to determine protein concentrations (34). AGP activity was determined by the pyrophosphorolysis assay (30).
Equivalent amounts of protein (4 μg) from heated and nonheated crude extracts were resolved through a 10% SDS/PAGE and blotted to a nitrocellulose membrane (35). SH2 and BT2 subunits were detected by using anti-SH2 and anti-BT2 antibody (35, 36).
Yeast Two-Hybrid Analysis.
Coding regions for WT Sh2 and Bt2 were previously cloned into the yeast two-hybrid expression vectors pGAD424 and pGBT9 (35). The coding region specific to Sh2hs33 was cloned in an identical fashion by using the primers designated for Sh2. The pSH2HS33-AD and pSH2HS33-BD constructs were confirmed by sequence analysis. Expression of pSH2HS33-AD/pBT2-BD and pSH2-AD/pBT2-BD in the yeast two-hybrid system followed previous methods (35). The strength of the subunit interactions was quantified by using β-galactosidase (β-gal) assays (35). The plasmids pVA3 and pTD1 that encode a murine p53 and simian virus 40 large T-antigen, respectively, were used as a positive control for a strong protein–protein interaction (CLONTECH).
Density Gradient Analysis.
Five to 30 percent glycerol gradients (5 ml) were prepared and run as previously described (30). Crude extracts were derived from 100 ml of Luria broth cultures. Equal volumes of 50 μl for each extract were centrifuged at 50,000 rpm for 20 hr at 4°C and fractionated at a flow rate of 1 ml/min. β-gal (540 kDa), calf intestinal alkaline phosphatase (CIAP) (140 kDa), and horseradish peroxidase (HRP) (44 kDa) were the molecular mass standards. AGP activity was monitored by using the pyrophosphorolysis assay (30). β-gal activity was monitored as described above. CIAP and HRP were monitored enzymatically (35). Aliquots (50 μl) from the individual fractions were diluted into 50 μl of denaturing solution (100 mM Tris-Cl, pH 6.8, 4% SDS, and 200 mM DTT) and boiled for 5 min. Twenty microliters of the denatured protein sample was dot-blotted to a nitrocellulose membrane, and the subunits were detected by using antibodies specific to SH2 and BT2 (35).
RESULTS
Isolation and Characterization of Heat-Stable Variants.
That expression of the WT Sh2 and Bt2 sequences exhibited a reduced capacity to complement the E. coli AGP mutant at the elevated temperature of 42°C allowed for selection of more stable variants of the maize endosperm AGP. Mutant Sh2 plasmid was transformed into AC70R1–504 cells containing the WT Bt2 plasmid. Isolates with enhanced ability to complement the mutant E. coli were identified by their rapid and dark iodine staining phenotypes when grown at the elevated temperature of 42°C. Eleven isolates were identified.
Enzyme studies of four mutants revealed an increased stability when extracts of bacterially expressed maize endosperm AGP were incubated at 60°C for 5 min (Fig. 1). This treatment reduced WT AGP activity approximately 75%. Percentage retention of AGP activity in the WT heated extract was slightly higher than previously reported (19) for the endosperm-expressed AGP. This finding is likely caused by the addition of 30% ammonium sulfate and 10 mM K-Pi to the extraction buffer. Inclusion of these reagents greatly increased the stability of the maize endosperm AGP in our hands. In agreement with previous reports (20, 21, 37–39), potato AGP is resistant to the 60°C/5-min treatment. More than 90% of the potato AGP activity remained after the heat treatment.
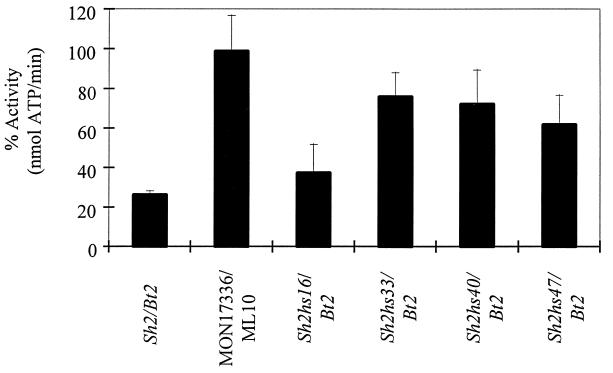
Figure 1.
AGP activity after heat treatment at 60°C for 5 min. WT (Sh2/Bt2) maize endosperm AGP lost 74% of its activity, whereas the heat-stable potato AGP (MON17336/ML10) retained more than 95% of its activity. AGP stability varied from 37% in the Sh2hs16/Bt2 extract to a maximum of 76% in the Sh2hs33/Bt2 extract. Results are the average of three independent enzyme preparations, and the standard error is shown.
Stability of the Sh2 mutants varied considerably. Sh2hs16 is least stable and retained only 37% of its AGP activity after heat treatment. Much higher activity was obtained with Sh2hs33 and Sh2hs40 (Fig. 1). Of significance, the specific activity of AGP in the crude extracts before heat treatment was consistently 2-fold higher in Sh2hs33 (0.43 unit/mg) and Sh2hs40 (0.43 unit/mg) extracts compared with WT (0.21 unit/mg). Sh2hs33 showed the greatest retention of AGP activity at 76% post-heat treatment.
Sequence Analysis of Heat-Stable Variants.
A single point mutation was identified in Sh2hs33. This mutation generated a His-to-Tyr mutation at amino acid position 333 in the Sh2 coding region (Fig. 2). The identical mutation was identified in Sh2hs39 (Table 1), and no additional characterization of this mutant was conducted. This His residue and the surrounding region is highly conserved in the heat-labile, cereal endosperm large subunits (40–43). Sequencing of additional heat-stable variants identified the His-to-Tyr change either alone or in combination with other changes in four of the seven mutants (Table 1). This result clearly identifies this residue as important in the stability of the maize endosperm AGP.
Figure 2.
Primary sequence comparisons between the AGP large subunits of maize endopserm (28), wheat seed (41), barley seed (42), rice endosperm (43), and potato tuber (44). Alignments show the His residue mutated in Sh2hs33 (bold), and the surrounding region is highly conserved among the cereal endosperm. There is a Tyr at the corresponding residue in the potato large subunit.
Table 1.
Mutational alterations identified from the sequence analysis of heat-stable mutants
| Mutation | |
|---|---|
| Sh2hs13 | Ala to Pro at position 177 |
| Sh2hs14 | Asp to His at position 400 |
| Val to Ile at position 454 | |
| Sh2hs16 | Arg to Thr at position 104 |
| Sh2hs33 | His to Tyr at position 333 |
| Sh2hs39 | His to Tyr at position 333 |
| Sh2hs40 | His to Tyr at position 333 |
| Thr to Ile at position 460 | |
| Sh2hs47 | Arg to Pro at position 217 |
| His to Tyr at position 333 |
The His-to-Tyr mutation was identified in four of the seven variants analyzed either alone or in combination with a second mutation.
Noteworthy is the fact that the large subunit of the heat-stable potato AGP contains a Tyr residue at the position corresponding to the His-to-Tyr mutation of Sh2hs33 (Fig. 2) (44). Hence, a single mutation of His to Tyr is sufficient to condition heat stability in the maize endosperm AGP.
Protein Analysis of Heat-Stable Variants.
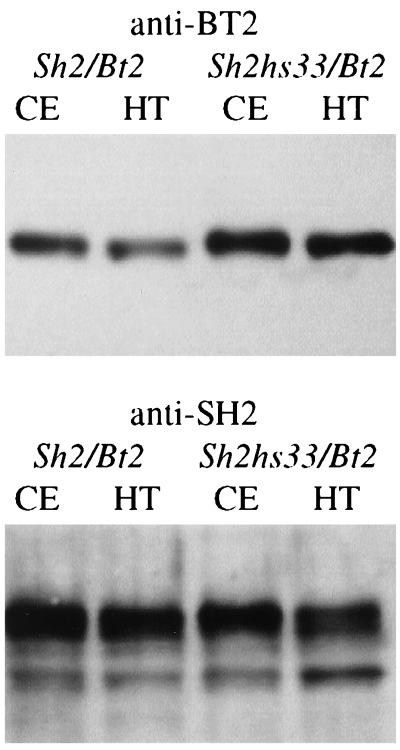
Heated and nonheated extracts from WT and Sh2hs33 were subjected to SDS/PAGE and protein blot analysis. Within each genotype, the heated and nonheated preparations were virtually identical. These data rule out mechanisms involving SH2 degradation as the cause of activity loss. Furthermore, comparable levels of SH2 antigen were identified between WT and Sh2hs33.
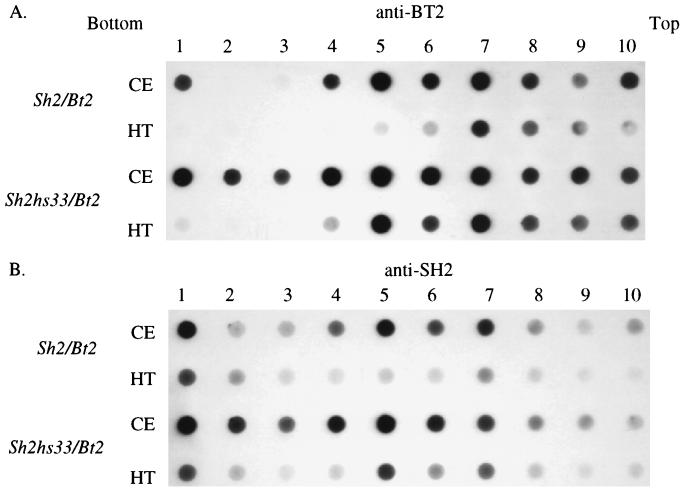
In contrast, a distinct difference between WT and Sh2hs33 was noted in the BT2 antigen amount (Fig. 3). This result was highly reproducible and also was found with the Sh2hs40 mutant (data not shown). Of significance, the Sh2hs40 mutant retained 72% of its AGP activity, which is comparable to Sh2hs33 (Fig. 1). These results show that while the mutational alteration increasing AGP stability lies in the SH2 protein, levels of the BT2 protein are elevated. Hence, mutation in the SH2 subunit alters the level or stability of the BT2 subunit, pointing to altered interactions between SH2 and BT2.
Figure 3.
Equivalent amounts of protein from nonheated (CE) and heated crude extracts (HT) were resolved through a 10% SDS/PAGE, blotted to nitrocellulose, and probed with antibody to either the BT2 or SH2 subunit as indicated above the blot. Results show that there is a significant increase in the level of BT2 antigen in the Sh2hs33/Bt2 mutant extract compared with the WT (Sh2/Bt2) extract. In contrast, comparable levels of SH2 antigen were detected in the Sh2/Bt2 and Sh2hs33/Bt2 nonheated and heated extracts. Results indicate the mutation in Sh2hs33 stabilizes or recruits the BT2 subunit more efficiently than does the WT SH2 protein.
Yeast Two-Hybrid Expression of the Sh2hs33 Variant.
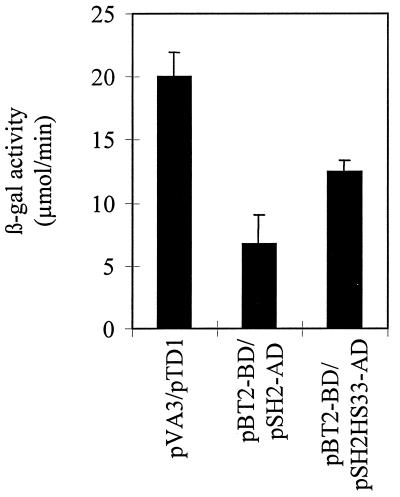
Cloning and expression of the heat-stable variant Sh2hs33 in the yeast two-hybrid expression system allowed us to further study the subunit interactions. Previously we showed that SH2 and BT2 interact in the yeast two-hybrid expression system (35). Likewise, expression of SH2HS33 and BT2 indicates that the two proteins interact as evidenced by a positive 5-bromo-4-chloro-3-indolyl β-d-galactoside staining phenotype (data not shown). No staining was detected when the SH2HS33 subunit was expressed alone. More importantly, the yeast two-hybrid system allowed us to quantify the interaction between the individual proteins by using β-gal assays. Expression of SH2HS33 and BT2 protein resulted in a β-gal activity of 12.47 μmol/min, or 62% of the positive control (Fig. 4). This finding is approximately a 2-fold increase over the β-gal activity observed with the expression of the WT SH2 with BT2 subunits (6.71 μmol/min) (Fig. 4). Results from the yeast two-hybrid expression system are consistent with the hypothesis that the mutation of a His to Tyr at position 333 on the SH2 subunit enhances the interaction with the BT2 subunit.
Figure 4.
β-gal assays were used to quantify the interactions between SH2 and SH2HS33 subunits and the WT BT2 subunit in the yeast two-hybrid system. Results from the assays clearly show an enhanced interaction between the SH2HS33 and BT2 (pBT2-BD/pSH2HS33) subunits compared with the expression of the WT SH2 (pBT2-BD/pSH2-AD) subunit. Results are the average of at least two independent assays, and the standard error is shown. β-gal activity from the expression of pVA3/pTD1 (CLONTECH) is included as a positive control.
Density Gradient Centrifugation.
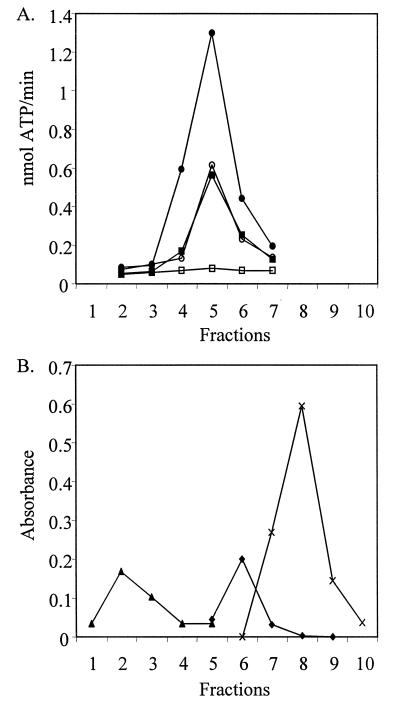
Glycerol density gradient analysis of heated and nonheated extracts of the bacterially expressed AGPs added additional biochemical support for the conclusion of increased AGP stability conditioned by Sh2hs33. Analysis of AGP activity from 5–30% glycerol gradients showed that the WT and mutant AGPs migrated in a range consistent with the molecular mass of the maize endosperm AGP (≈200 kDa) (Fig. 5). All preparations exhibited indistinguishable activity profiles. Hence heat treatment or the alteration in AGP subunit interactions induced by Sh2hs33 does not alter the aggregation state of the active enzyme (Fig. 5).
Figure 5.
(A) Enzyme analysis of fractions from a 5–30% glycerol gradient of nonheated (■) and heated (□) crude extract from WT and nonheated (•) and heated (○) crude extract from the Sh2hs33 mutant expressed in E. coli. AGP activity profiles of WT and Sh2hs33 nonheated extracts are identical and are plotted on the y-axis. However, heat treatment completely abolishes AGP activity in the WT. Fractions 1–10 are labeled on the x-axis and represent fractions from the bottom to the top of the gradient. (B) β-gal (540 kDa) (▴), calf intestinal alkaline phosphatase (140 kDa) (♦), and horseradish peroxidase (44 kDa) (X) are included as molecular mass standards and are plotted on the y-axis in absorbance at their respective wavelength.
Antibody analysis of gradient fractions from the heated and nonheated samples reveals that Sh2hs33 stabilizes heterotetrameric AGP formation (Fig. 6 A and B). BT2 antigen peaked with activity in Sh2/Bt2 (WT) and Sh2hs33/Bt2 nonheated samples (Figs. 5 and 6A). However, the BT2 antigen disappears in the WT fraction 5 after heat treatment whereas significant levels of BT2 antigen remain in Sh2hs33/Bt2 fraction 5. Interestingly, a peak of BT2 antigen in the 100-kDa range is virtually unaffected by the heat treatment in the WT and mutant. Similar results were noted when the blot was probed with the anti-SH2 antibody. No significant level of SH2 is detected in the heated WT extract, whereas SH2 antigen still peaks in fraction 5 in Sh2hs33/Bt2. These results provide direct evidence for enhanced subunit interactions in the Sh2hs33 mutant and confirm the mutational stabilization of the heterotetrameric complex of maize endosperm AGP.
Figure 6.
Protein blot analysis of 5–30% glycerol density gradient fractions. Blots were probed with antibody to either BT2 or SH2 as indicated. A peak of BT2 and SH2 protein is identified in fraction 5 in the nonheated (CE) extracts of Sh2/Bt2 and Sh2hs33/Bt2, a result consistent with the peak of AGP activity (Fig. 5A). In the heated extract (HT) of Sh2/Bt2, BT2 and SH2 antigen levels are abolished in fraction 5, which contains the peak of AGP activity, whereas significant levels of BT2 and SH2 remain in the heated Sh2hs33/Bt2 extract. This finding indicates the mutation in Sh2hs33 not only enhances the interaction with the BT2 subunit, but in turn, stabilizes the tetrameric nature of AGP.
DISCUSSION
The aim of this work was to use the advantages of E. coli genetics to isolate agriculturally useful variants in a plant gene. We focused on AGP, a rate-limiting step in starch biosynthesis, and isolated variants with increased heat stability. Temperature lability of AGP likely represents a major limitation to dry weight deposition when kernels develop at elevated temperatures.
Among the various mutants isolated, a His-to-Tyr mutation at amino acid position 333 was repeatedly found. Although this position of the protein has not yet been associated with catalytic or allosteric functions, we note that the heat-stable potato AGP contains a Tyr residue at this position. Clearly this position is important in heat stability. It is interesting to note that the Arg-to-Thr mutation of Sh2hs16 corresponds to the Lys-39 residue of the E. coli AGP. This residue is at the activator binding site within E. coli AGP (45). Although the change at position 177 in Sh2hs13 was found only once, we recently isolated a second-site suppressor of a cold-sensitive maize endosperm AGP mutant (unpublished work) at exactly this residue. Hence, this site is likely quite important in SH2 and BT2 interactions. The mutation of Thr to Ile identified in Sh2hs40 appears to be less significant because this mutant shows a similar level of stability at the enzyme level. In the Sh2hs47 mutant, the additional Arg-to-Pro mutation at position 217 of Sh2 appears to be antagonistic to the His-to-Tyr mutation because the AGP stability is negatively impacted compared with Sh2hs33 (Fig. 1).
Of particular interest, all of the single-site mutations conditioning heat stability involve changes in endosperm-conserved amino acids. Furthermore, we show that enhanced heat stability of AGP can arise via various mutations. These data raise the interesting possibility that heat lability of endosperm AGP may have been a trait selected for during the evolution of these cereals. In this regard we note that the maize embryo AGP is quite heat stable relative to the endosperm AGP (18, 19). Hence, under conditions of heat stress, the differential lability of the seed AGPs may favor the flow of carbon to the embryo, perhaps providing a selective advantage to the seed in subsequent germination.
The results presented here are significant on several levels. First, the heat-stable variants of maize endosperm AGP may provide for superior manipulation of the flux of carbon into starch in maize kernels. Precedence for the use of superior endosperm AGPs and their affect on yield recently have been described (17). Second, the variant AGPs may have a significant role in alleviating the heat-induced yield loss observed when maize kernels are grown at elevated temperatures. Isolation of the stability mutants such as Sh2hs33 provides us with the tools necessary to decipher AGPs role in this significant agricultural problem. It is also noteworthy that Sh2hs33 conditions enhanced activity before heat treatment. Hence, this mutation may have utility at temperatures not considered stressful for plant agriculture. Furthermore, comparison of developmental profiles of AGP activity versus SH2 and BT2 antigen points to the fact that activity is lost before degradation of the enzyme subunits (46, 47). Hence, Sh2hs33 may extend the developmental profile of AGP activity. Transgenic studies with Sh2hs33 are currently underway. Third, the heat-stable variants provide initial information concerning SH2 and BT2 subunit interactions. These mutants, coupled with the recent demonstration of expression of SH2 and BT2 subunits in a yeast two-hybrid expression system (35), provide powerful tools to decipher sites of interaction between the two subunits.
Heterologous expression of various plant AGPs is beginning to answer some basic questions concerning AGP function and its allosteric properties (30, 33, 37–39, 48, 49). There still remain, however, voids in our understanding, particularly in how the individual subunits interact to form active tetramers. Taken as a whole, the work here identifies a stability mutant of an endosperm AGP and clearly defines the importance of subunit interactions. These mutants also point to sites of interaction between the SH2 and BT2 subunits of maize endosperm AGP.
Acknowledgments
We thank Dr. Karen Koch for discussions concerning the evolutionary significance of heat labile AGP. Synthesis of DNA primers and DNA sequencing were done in the facilities of the Interdisciplinary Center for Biotechnology Research at the University of Florida. We gratefully acknowledge extramural support from the National Science Foundation (IBN-9316887 and MCB-9420422), U.S. Department of Agriculture/National Research Initiative (94-7300-453, 97-36306-4461, 95-37301-2080, and 98-1006), and Pioneer Hi-Bred International. This is Florida Agricultural Experiment Station Journal Series No. R-06417.
ABBREVIATIONS
- AGP
ADP-glucose pyrophosphorylase
- β-gal
β-galactosidase
- Bt2
Brittle2
- Sh2
Shrunken2
- WT
wild type
References
- 1. Hunter R B, Tollenaar M, Breuer C M. Can J Plant Sci. 1977;57:1127–1133. [Google Scholar]
- 2.Jones R J, Gengenbach B G, Cardwell V B. Crop Sci. 1981;21:761–766. [Google Scholar]
- 3.Chowdhury S I, Wardlaw I F. Aust J Agric Res. 1978;29:205–223. [Google Scholar]
- 4.Badu-Apraku B, Hunter R B, Tollenaar M. Can J Plant Sci. 1983;63:357–363. [Google Scholar]
- 5.Wardlaw I F, Moncur L. Aust J Plant Physiol. 1995;22:391–397. [Google Scholar]
- 6.Corbellini M, Canevar M G, Mazza L, Ciaffi M, Lafiandra D, Borghi B. Aust J Plant Physiol. 1997;24:245–260. [Google Scholar]
- 7.Thompson L M. Science. 1975;188:535–541. doi: 10.1126/science.188.4188.535. [DOI] [PubMed] [Google Scholar]
- 8.Conroy J P, Seneweera S, Basra A S, Rogers G, Nissen-Wooller B. Aust J Plant Physiol. 1994;21:741–758. [Google Scholar]
- 9.Cheikn N, Jones R J. Physiol Plant. 1995;95:59–66. [Google Scholar]
- 10.Jones R J, Ouattar S, Crookston R K. Crop Sci. 1984;24:133–137. [Google Scholar]
- 11.Bhullar S S, Jenner C F. Aust J Plant Physiol. 1986;13:617–626. [Google Scholar]
- 12.Singletary G W, Banisadr R, Keeling P L. Aust J Plant Physiol. 1994;21:829–841. doi: 10.1104/pp.113.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallwork M A B, Logue S J, MacLeod L C, Jenner C F. Aust J Plant Physiol. 1998;25:173–181. [Google Scholar]
- 14.Preiss J, Romeo T. Progr Nucleic Acid Res Mol Biol. 1994;47:299–329. doi: 10.1016/s0079-6603(08)60255-x. [DOI] [PubMed] [Google Scholar]
- 15.Hannah L C. In: Cellular and Molecular Biology of Plant Seed Development. Larkins B A, Vasil I K, editors. Dordrecht, The Netherlands: Kluwer; 1997. pp. 375–405. [Google Scholar]
- 16.Stark D M, Timmerman K, Barry G F, Preiss J, Kishore G M. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- 17.Giroux M J, Shaw J, Barry G, Cobb G B, Greene T, Okita T W, Hannah L C. Proc Natl Acad Sci USA. 1996;93:5824–5829. doi: 10.1073/pnas.93.12.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preiss J, Lammel C, Sabraw A. Plant Physiol. 1971;47:104–108. doi: 10.1104/pp.47.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannah L C, Tuschall D M, Mans R J. Genetics. 1980;95:961–970. doi: 10.1093/genetics/95.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowokinos J R, Preiss J. Plant Physiol. 1982;69:1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okita T W, Nakata P A, Anderson J M, Sowokinos J, Morell J, Preiss J. Plant Physiol. 1990;93:785–790. doi: 10.1104/pp.93.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duke E R, Doehlert D C. Environ Exp Botany. 1996;36:199–208. [Google Scholar]
- 23.Hawker J S, Jenner C F. Aust J Plant Physiol. 1993;20:197–209. [Google Scholar]
- 24.Keeling P L, Bacon P J, Holt D C. Planta. 1993;191:342–348. [Google Scholar]
- 25.Denyer K, Hylton C M, Smith A M. Aust J Plant Physiol. 1994;21:783–789. [Google Scholar]
- 26.Keeling P L, Banisadr R, Barone L, Wasserman B P, Singletary G W. Aust J Plant Physiol. 1994;21:807–827. [Google Scholar]
- 27.Hannah L C, Nelson O E., Jr Biochem Genet. 1976;14:547–560. doi: 10.1007/BF00485834. [DOI] [PubMed] [Google Scholar]
- 28.Bhave M R, Lawrence S, Barton C, Hannah L C. Plant Cell. 1990;2:581–588. doi: 10.1105/tpc.2.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae J M, Giroux M, Hannah L C. Maydica. 1990;35:317–322. [Google Scholar]
- 30.Greene T W, Chantler S E, Kahn M L, Barry G F, Preiss J, Okita T W. Proc Natl Acad Sci USA. 1996;93:1509–1513. doi: 10.1073/pnas.93.4.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iglesias A, Barry G F, Meyer C, Bloksberg L, Nakata P, Greene T, Laughlin M J, Okita T W, Kishore G M, Preiss J. J Biol Chem. 1993;268:1081–1086. [PubMed] [Google Scholar]
- 32.Govons S, Vinopal R, Ingraham J, Preiss J. J Bacteriol. 1969;97:970–972. doi: 10.1128/jb.97.2.970-972.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene T W, Woodbury R L, Okita T W. Plant Physiol. 1996;112:1315–1320. doi: 10.1104/pp.112.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Greene T W, Hannah L C. Plant Cell. 1998;10:1295–1306. doi: 10.1105/tpc.10.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giroux M J, Hannah L C. Mol Gen Genet. 1994;243:400–408. doi: 10.1007/BF00280470. [DOI] [PubMed] [Google Scholar]
- 37.Okita T W, Greene T W, Laughlin M J, Salamone P, Woodbury R, Choi S, Ito H, Kavakli H, Stephens K. In: Engineering Crops for Industrial End Uses. Shewry P R, Napier J A, Davis P, editors. London: Portland Press; 1998. , in press. [Google Scholar]
- 38.Laughlin M J, Payne J, Okita T W. Phytochemistry. 1998;47:621–629. doi: 10.1016/s0031-9422(97)00578-5. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin M J, Chantler S E, Okita T W. Plant J. 1998;14:159–168. doi: 10.1046/j.1365-313x.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith-White B J, Preiss J. J Mol Evol. 1992;34:449–464. doi: 10.1007/BF00162999. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth C, Hosein F, Tarvis M, Weir F, Burrell M, Devos K M, Gale M D. Planta. 1995;197:1–10. doi: 10.1007/BF00239933. [DOI] [PubMed] [Google Scholar]
- 42.Villand P, Olsen O-A, Lilian A, Kleczkowski L A. Plant Physiol. 1992;100:1617–1618. doi: 10.1104/pp.100.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavakli I H, Wu Y, Okita T W. Plant Physiol. 1997;112:1399. [Google Scholar]
- 44.Nakata P A, Greene T W, Anderson J W, Smith-White B J, Okita T W, Preiss J. Plant Mol Biol. 1991;17:1089–1093. doi: 10.1007/BF00037149. [DOI] [PubMed] [Google Scholar]
- 45.Meyer C R, Ghosh P, Nadler S, Preiss J. Arch Biochem Biophys. 1993;302:64–71. doi: 10.1006/abbi.1993.1181. [DOI] [PubMed] [Google Scholar]
- 46.Giroux M J, Boyer C, Feix G, Hannah L C. Plant Physiol. 1994;106:713–722. doi: 10.1104/pp.106.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prioul J L, Jeanette E, Reyss A, Gregory N, Giroux M, Hannah L C, Causse M. Plant Physiol. 1994;104:179–187. doi: 10.1104/pp.104.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greene T W, Kavakli I H, Kahn M L, Okita T W. Proc Natl Acad Sci USA. 1998;95:10322–10327. doi: 10.1073/pnas.95.17.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudi H, Doan D N P, Olaen O-A. FEBS Lett. 1997;419:124–130. doi: 10.1016/s0014-5793(97)01448-8. [DOI] [PubMed] [Google Scholar]