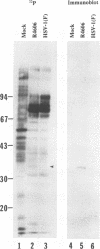
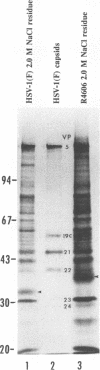
Abstract
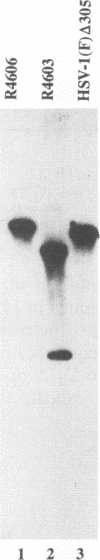
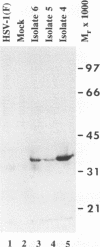
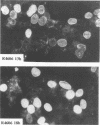
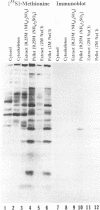
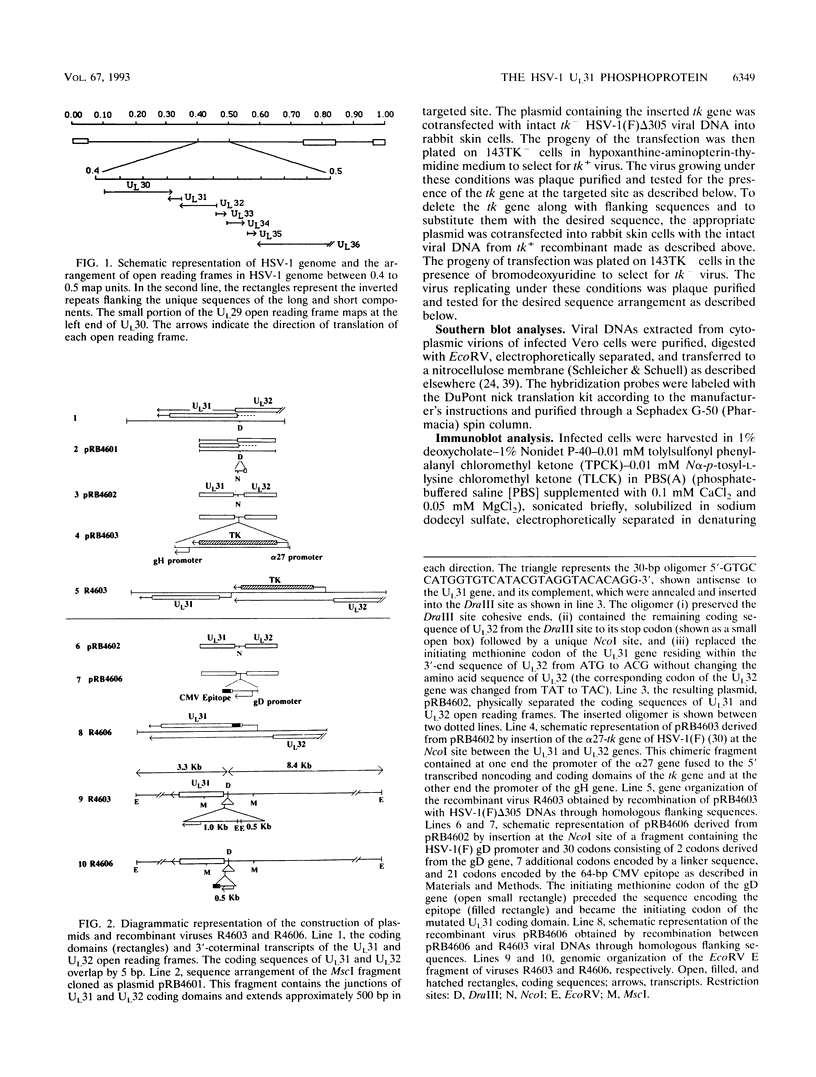
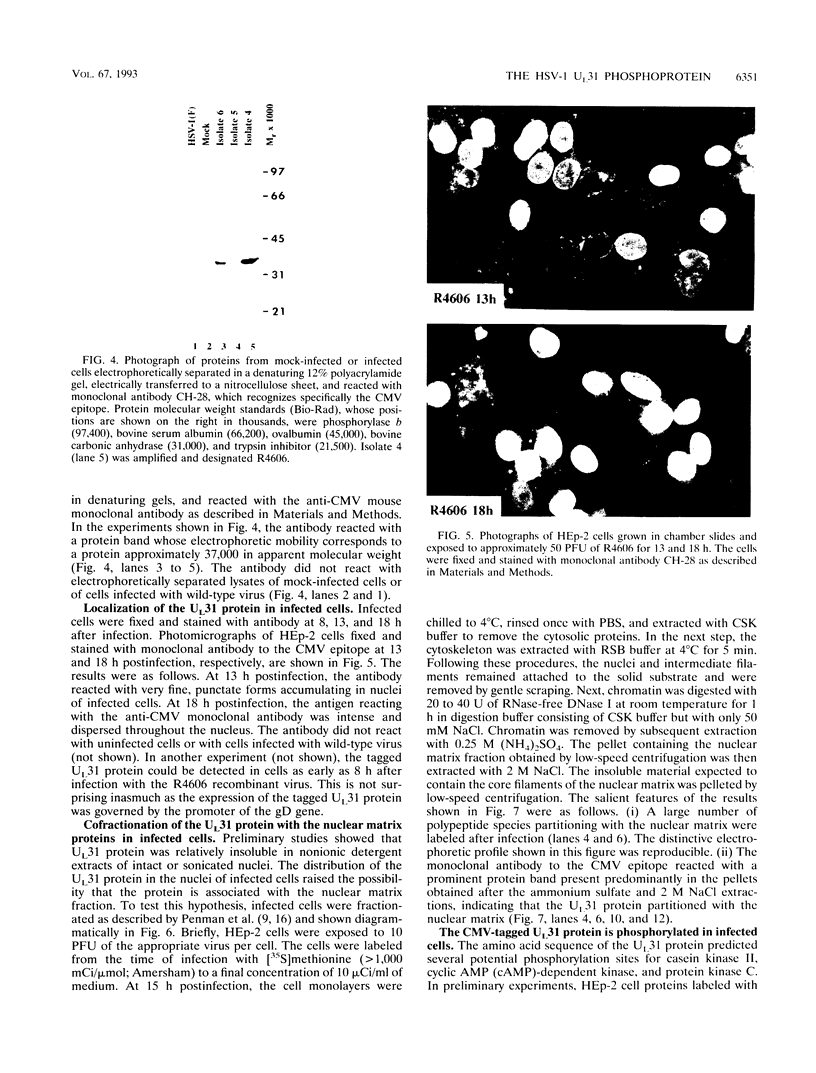
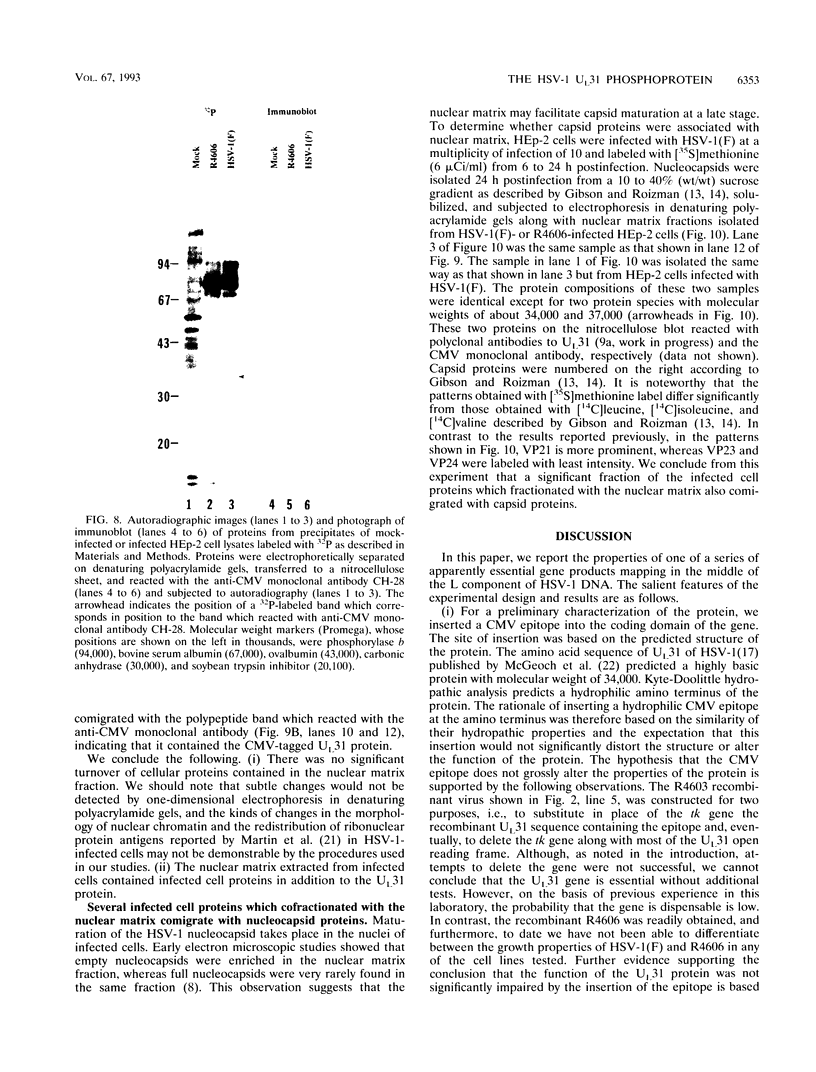
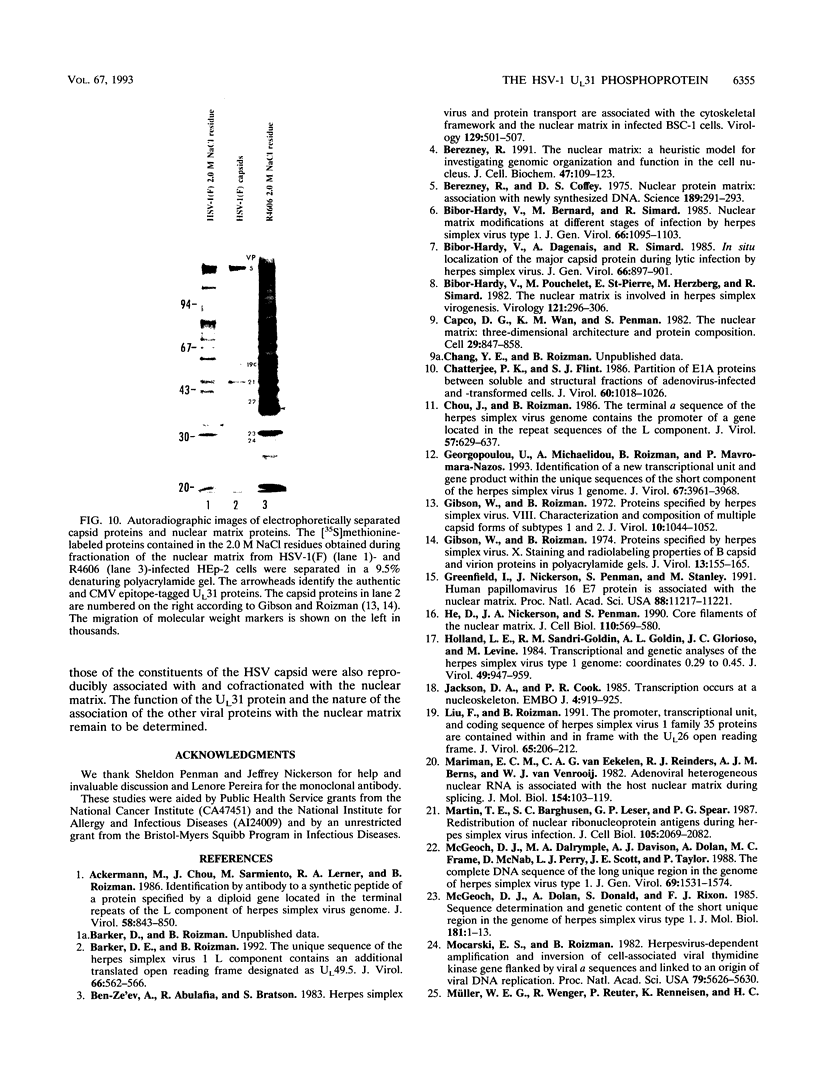
The nucleotide sequence of the UL31 open reading frame is predicted to encode a basic protein with a hydrophilic amino terminus and a nuclear localization signal. To identify its gene product, we constructed a viral genome in which the thymidine kinase gene was inserted between the UL31 and UL32 open reading frames. The thymidine kinase gene was then deleted, and in the process, the 5' terminus of the UL31 open reading frame was replaced with a 64-bp sequence in frame with the complete, authentic sequence of the UL31 open reading frame. The inserted sequence encoded a hydrophilic epitope derived from glycoprotein B of human cytomegalovirus and for which a monoclonal antibody is available. We report that in infected cells, the tagged protein localized in and was dispersed throughout the nucleus. Nuclear fractionation studies revealed that the UL31 protein partitions with the nuclear matrix. The protein is phosphorylated in infected cells maintained in medium containing 32Pi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Chou J., Sarmiento M., Lerner R. A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986 Jun;58(3):843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. The unique sequence of the herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J Virol. 1992 Jan;66(1):562–566. doi: 10.1128/jvi.66.1.562-566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Bratosin S. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology. 1983 Sep;129(2):501–507. doi: 10.1016/0042-6822(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Berezney R. The nuclear matrix: a heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991 Oct;47(2):109–123. doi: 10.1002/jcb.240470204. [DOI] [PubMed] [Google Scholar]
- Bibor-Hardy V., Bernard M., Simard R. Nuclear matrix modifications at different stages of infection by herpes simplex virus type 1. J Gen Virol. 1985 May;66(Pt 5):1095–1103. doi: 10.1099/0022-1317-66-5-1095. [DOI] [PubMed] [Google Scholar]
- Bibor-Hardy V., Dagenais A., Simard R. In situ localization of the major capsid protein during lytic infection by herpes simplex virus. J Gen Virol. 1985 Apr;66(Pt 4):897–901. doi: 10.1099/0022-1317-66-4-897. [DOI] [PubMed] [Google Scholar]
- Bibor-Hardy V., Pouchelet M., St-Pierre E., Herzberg M., Simard R. The nuclear matrix is involved in herpes simplex virogenesis. Virology. 1982 Sep;121(2):296–306. doi: 10.1016/0042-6822(82)90169-6. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Partition of E1A proteins between soluble and structural fractions of adenovirus-infected and -transformed cells. J Virol. 1986 Dec;60(3):1018–1026. doi: 10.1128/jvi.60.3.1018-1026.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J Virol. 1986 Feb;57(2):629–637. doi: 10.1128/jvi.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulou U., Michaelidou A., Roizman B., Mavromara-Nazos P. Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J Virol. 1993 Jul;67(7):3961–3968. doi: 10.1128/jvi.67.7.3961-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield I., Nickerson J., Penman S., Stanley M. Human papillomavirus 16 E7 protein is associated with the nuclear matrix. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11217–11221. doi: 10.1073/pnas.88.24.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D. C., Nickerson J. A., Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990 Mar;110(3):569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991 Jan;65(1):206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Barghusen S. C., Leser G. P., Spear P. G. Redistribution of nuclear ribonucleoprotein antigens during herpes simplex virus infection. J Cell Biol. 1987 Nov;105(5):2069–2082. doi: 10.1083/jcb.105.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Herpesvirus-dependent amplification and inversion of cell-associated viral thymidine kinase gene flanked by viral a sequences and linked to an origin of viral DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5626–5630. doi: 10.1073/pnas.79.18.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Wenger R., Reuter P., Renneisen K., Schröder H. C. Association of Tat protein and viral mRNA with nuclear matrix from HIV-1-infected H9 cells. Biochim Biophys Acta. 1989 Jul 7;1008(2):208–212. doi: 10.1016/0167-4781(80)90011-1. [DOI] [PubMed] [Google Scholar]
- Nickerson J. A., Krockmalnic G., Wan K. M., Turner C. D., Penman S. A normally masked nuclear matrix antigen that appears at mitosis on cytoskeleton filaments adjoining chromosomes, centrioles, and midbodies. J Cell Biol. 1992 Feb;116(4):977–987. doi: 10.1083/jcb.116.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard M. F., Simard R., Bibor-Hardy V. DNA-binding proteins of herpes simplex virus type 1-infected BHK cell nuclear matrices. J Gen Virol. 1987 Mar;68(Pt 3):727–735. doi: 10.1099/0022-1317-68-3-727. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Spector D., Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991 Nov;65(11):5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves F. C., Spector D., Roizman B. UL34, the target of the herpes simplex virus U(S)3 protein kinase, is a membrane protein which in its unphosphorylated state associates with novel phosphoproteins. J Virol. 1992 Jul;66(7):4295–4303. doi: 10.1128/jvi.66.7.4295-4303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Nuclear subcompartmentalization of simian virus 40 large T antigen: evidence for in vivo regulation of biochemical activities. J Virol. 1989 May;63(5):2308–2316. doi: 10.1128/jvi.63.5.2308-2316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Parent A., Silverstein S., Efstratiadis A. Pre-mRNA splicing and the nuclear matrix. Mol Cell Biol. 1987 Jan;7(1):111–120. doi: 10.1128/mcb.7.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z. H., Nickerson J. A., Krochmalnic G., Penman S. Alterations in nuclear matrix structure after adenovirus infection. J Virol. 1987 Apr;61(4):1007–1018. doi: 10.1128/jvi.61.4.1007-1018.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]