Abstract
The ability of enveloped viruses to cause disease depends on their ability to enter the host cell via membrane fusion events. An understanding of these early events in infection, crucial for the design of methods of blocking infection, is needed for viruses that mediate membrane fusion at neutral pH, such as paramyxoviruses and human immunodeficiency virus. Sialic acid is the receptor for the human parainfluenza virus type 3 (HPF3) hemagglutinin-neuraminidase (HN) glycoprotein, the molecule responsible for binding of the virus to cell surfaces. In order for the fusion protein (F) of HPF3 to promote membrane fusion, the HN must interact with its receptor. In the present report, two variants of HPF3 with increased fusion-promoting phenotypes were selected and used to study the function of the HN glycoprotein in membrane fusion. Increased fusogenicity correlated with single amino acid changes in the HN protein that resulted in increased binding of the variant viruses to the sialic acid receptor. These results suggest that the avidity of binding of the HN protein to its receptor regulates the level of F protein-mediated fusion and begin to define one role of the receptor-binding protein of a paramyxovirus in the membrane fusion process.
Full text
PDF
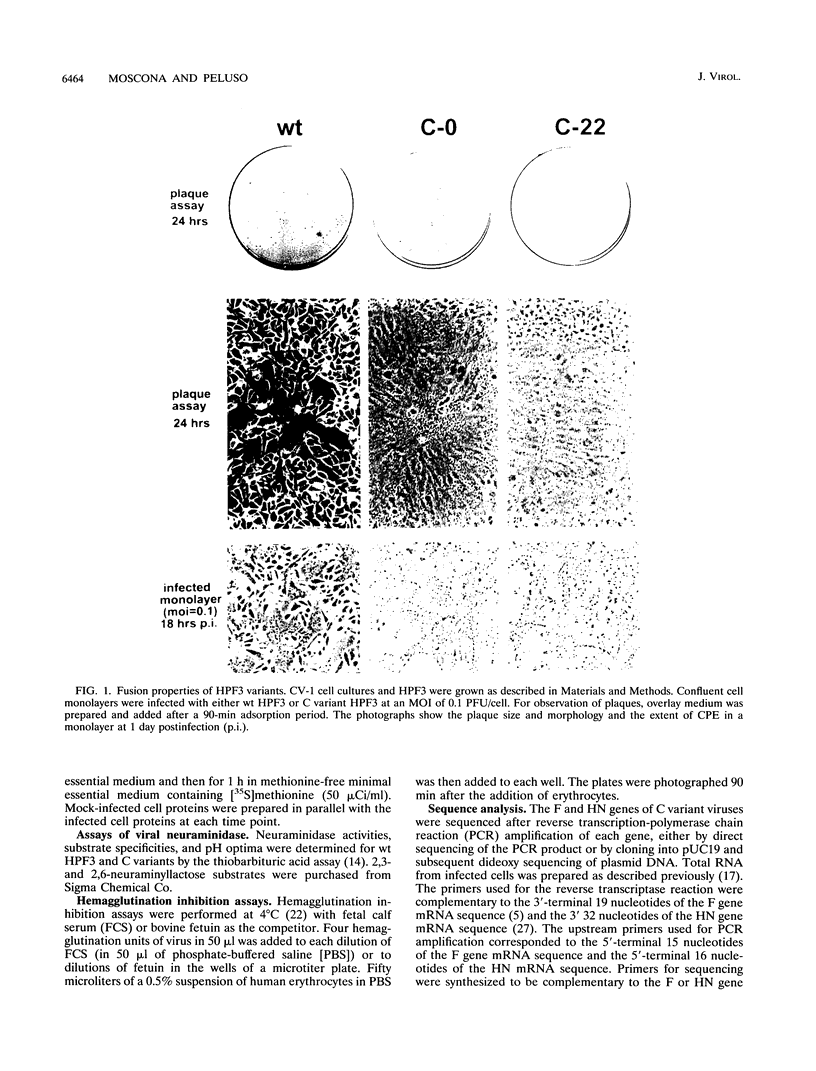

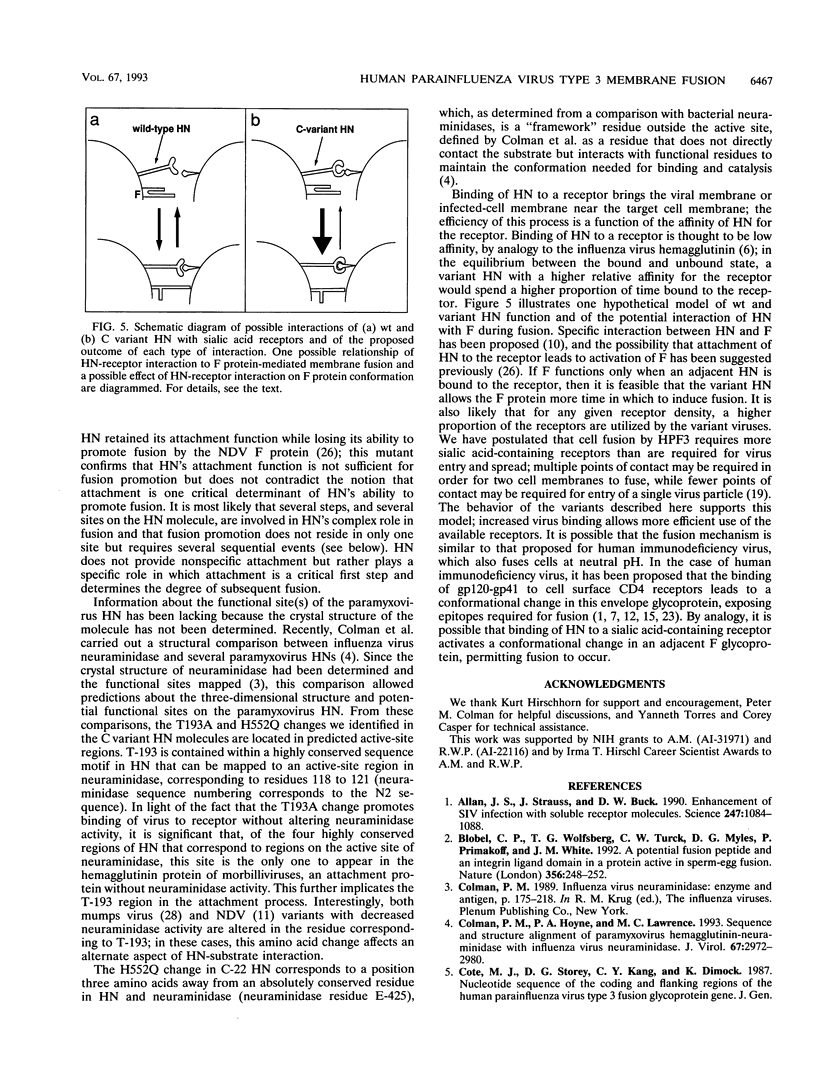

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Strauss J., Buck D. W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990 Mar 2;247(4946):1084–1088. doi: 10.1126/science.2309120. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Hoyne P. A., Lawrence M. C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993 Jun;67(6):2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. E., Sauter N. K., Skehel J. J., Wiley D. C. Proton nuclear magnetic resonance studies of the binding of sialosides to intact influenza virus. Virology. 1992 Aug;189(2):525–533. doi: 10.1016/0042-6822(92)90576-b. [DOI] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Paterson R. G., Shaughnessy M. A., Wood R., Lamb R. A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J Virol. 1992 Jul;66(7):4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. L., Ray R., Compans R. W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992 Mar;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Syddall R. J., Glickman R. L., Riel A. M., Sheehan J. P., Bratt M. A. Identification of amino acid residues important to the neuraminidase activity of the HN glycoprotein of Newcastle disease virus. Virology. 1989 Nov;173(1):196–204. doi: 10.1016/0042-6822(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Prehm P., Scheid A., Choppin P. W. Inhibition of the neuraminidase of paramyxoviruses by halide ions: a possible means of modulating the two activities of the HN protein. Virology. 1981 Jul 15;112(1):296–305. doi: 10.1016/0042-6822(81)90635-8. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Galinski M. S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J Virol. 1990 Jul;64(7):3212–3218. doi: 10.1128/jvi.64.7.3212-3218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992 Nov;66(11):6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Persistent infection with human parainfluenza virus 3 in CV-1 cells: analysis of the role of defective interfering particles. Virology. 1993 May;194(1):399–402. doi: 10.1006/viro.1993.1275. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Pritchett T. J., Lane J. L., Paulson J. C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983 Dec;131(2):394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Sergel T., McGinnes L. W., Peeples M. E., Morrison T. G. The attachment function of the Newcastle disease virus hemagglutinin-neuraminidase protein can be separated from fusion promotion by mutation. Virology. 1993 Apr;193(2):717–726. doi: 10.1006/viro.1993.1180. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Côté M. J., Dimock K., Kang C. Y. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus 3 hemagglutinin-neuraminidase gene: comparison with other paramyxoviruses. Intervirology. 1987;27(2):69–80. doi: 10.1159/000149722. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J. Identification of amino acids involved in the sialidase activity of the mumps virus hemagglutinin-neuraminadase protein. Virology. 1988 Nov;167(1):226–232. doi: 10.1016/0042-6822(88)90072-4. [DOI] [PubMed] [Google Scholar]