Abstract
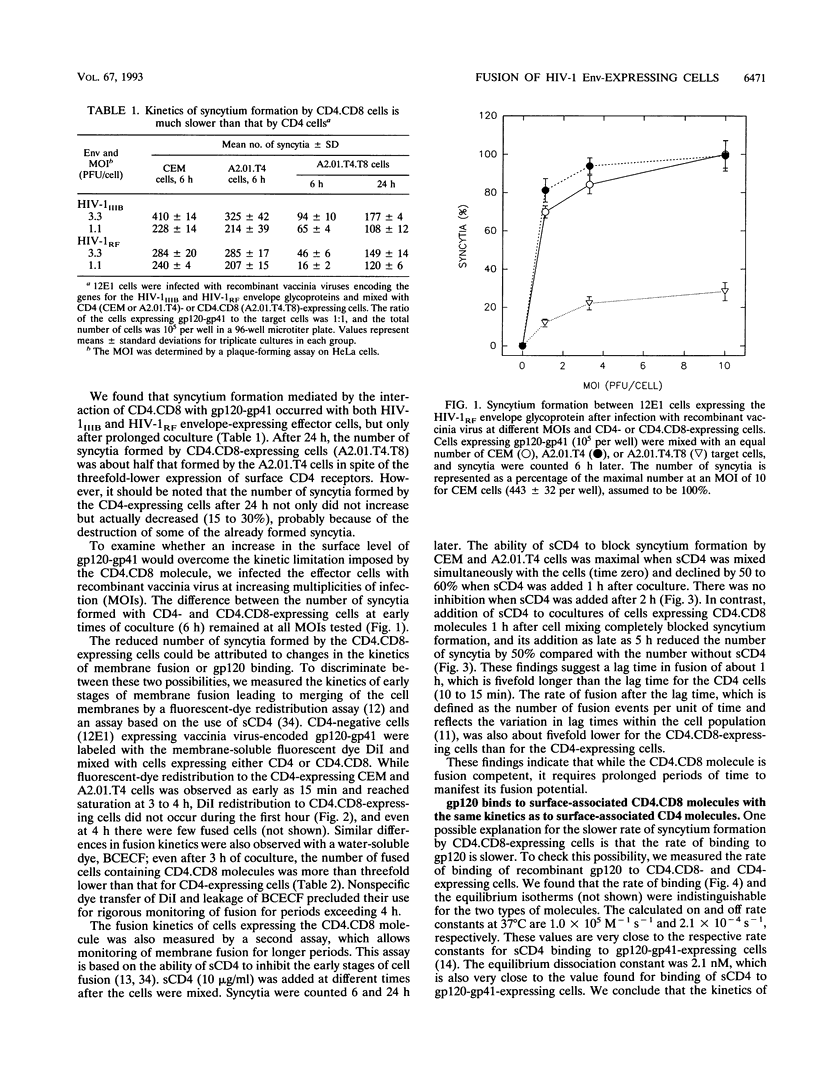
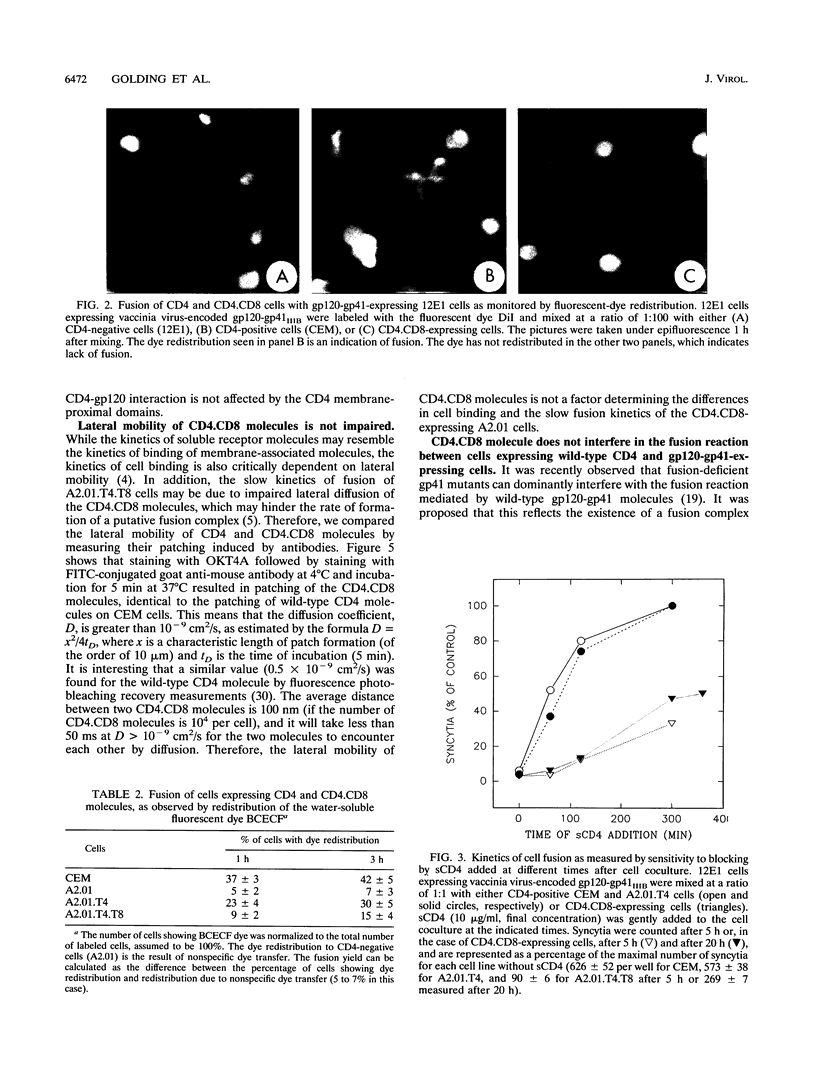
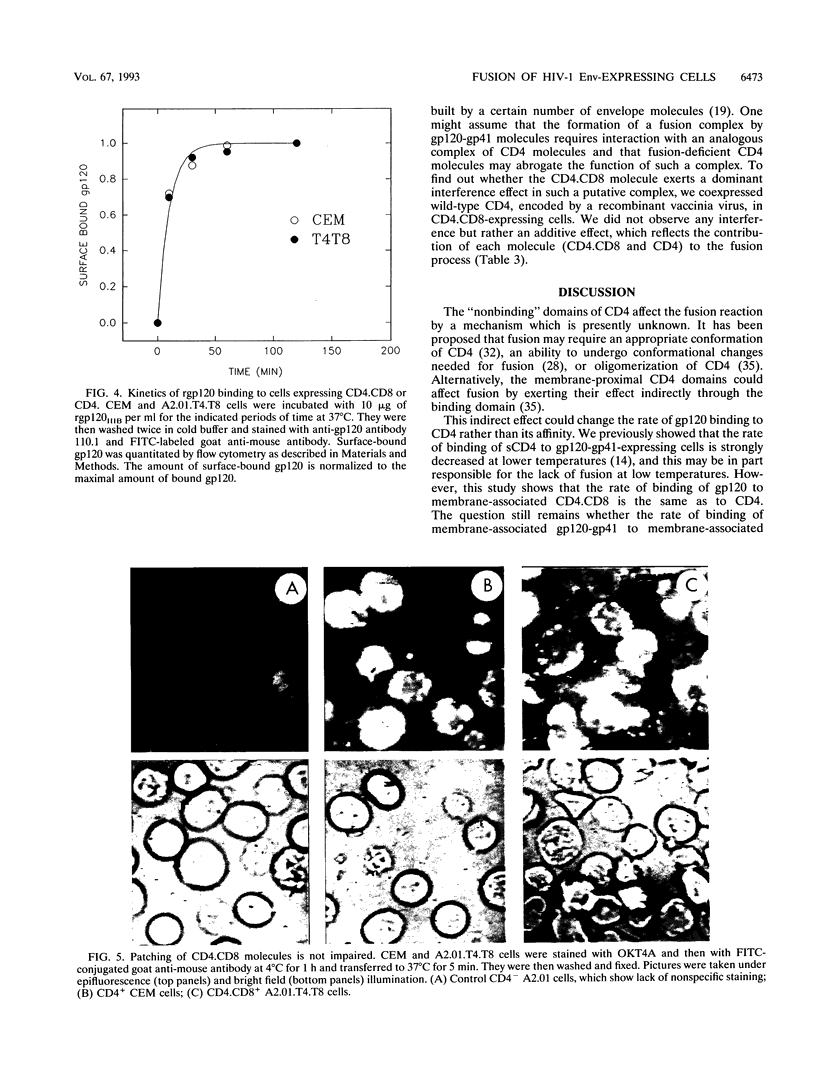
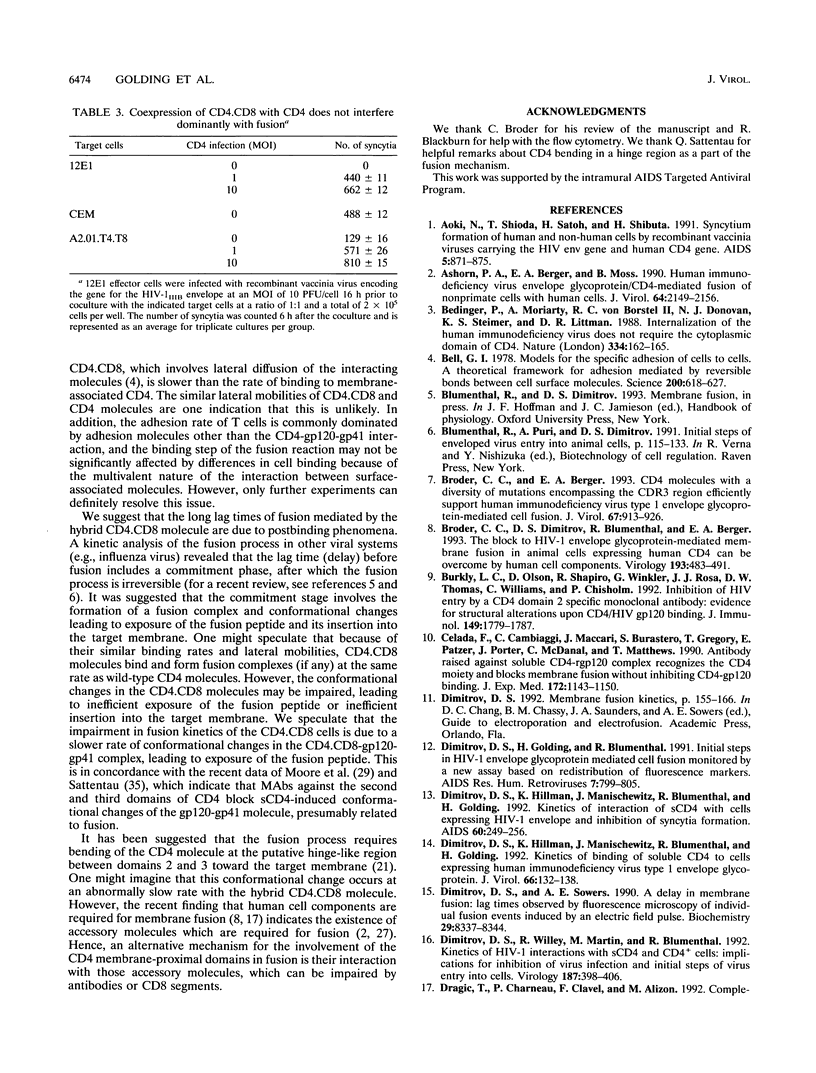
Several domains of CD4 have been suggested to play a critical role in events that follow its binding to the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (gp120-gp41). It has been reported previously that cells expressing a chimeric molecule consisting of the first 177 residues of human CD4 attached to residues from the hinge, transmembrane, and cytoplasmic domains of human CD8 did not form syncytia with HIV-1-infected cells (L. Poulin, L.A. Evans, S. Tang, A. Barboza, H. Legg, D.R. Littman, and J.A. Levy, J. Virol. 65: 4893-4901, 1991). In contrast, we found that the hybrid CD4.CD8 molecule expressed in human cells did render them susceptible to fusion with cells expressing HIV-1IIIB or HIV-1RF envelope glycoproteins encoded by vaccinia virus recombinants, but only after long lag times. The lag time of membrane fusion mediated by the hybrid CD4.CD8 molecule was fivefold longer than that for the wild-type CD4 molecule. However, the rate of binding to and the affinity of soluble gp120 for membrane-associated CD4.CD8 were the same as for CD4. Both molecules were laterally mobile, as determined by patching experiments. Coexpression of the CD4.CD8 chimera with wild-type CD4 did not lead to interference in fusion but had an additive effect. Therefore, the proximal membrane domains of CD4 play an important role in determining the kinetics of postbinding events leading to membrane fusion. We hypothesize that the long lag time is due to the inability of the CD4.CD8-gp120-gp41 complex to undergo the rapid conformational changes which occur during the fusion mediated by wild-type CD4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Shioda T., Satoh H., Shibuta H. Syncytium formation of human and non-human cells by recombinant vaccinia viruses carrying the HIV env gene and human CD4 gene. AIDS. 1991 Jul;5(7):871–875. doi: 10.1097/00002030-199107000-00012. [DOI] [PubMed] [Google Scholar]
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P., Moriarty A., von Borstel R. C., 2nd, Donovan N. J., Steimer K. S., Littman D. R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988 Jul 14;334(6178):162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Broder C. C., Berger E. A. CD4 molecules with a diversity of mutations encompassing the CDR3 region efficiently support human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion. J Virol. 1993 Feb;67(2):913–926. doi: 10.1128/jvi.67.2.913-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C. C., Dimitrov D. S., Blumenthal R., Berger E. A. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology. 1993 Mar;193(1):483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Olson D., Shapiro R., Winkler G., Rosa J. J., Thomas D. W., Williams C., Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol. 1992 Sep 1;149(5):1779–1787. [PubMed] [Google Scholar]
- Celada F., Cambiaggi C., Maccari J., Burastero S., Gregory T., Patzer E., Porter J., McDanal C., Matthews T. Antibody raised against soluble CD4-rgp120 complex recognizes the CD4 moiety and blocks membrane fusion without inhibiting CD4-gp120 binding. J Exp Med. 1990 Oct 1;172(4):1143–1150. doi: 10.1084/jem.172.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Golding H., Blumenthal R. Initial stages of HIV-1 envelope glycoprotein-mediated cell fusion monitored by a new assay based on redistribution of fluorescent dyes. AIDS Res Hum Retroviruses. 1991 Oct;7(10):799–805. doi: 10.1089/aid.1991.7.799. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Hillman K., Manischewitz J., Blumenthal R., Golding H. Correlation between kinetics of soluble CD4 interactions with HIV-1-Env-expressing cells and inhibition of syncytia formation: implications for mechanisms of cell fusion and therapy for AIDS. AIDS. 1992 Mar;6(3):249–256. doi: 10.1097/00002030-199203000-00001. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Hillman K., Manischewitz J., Blumenthal R., Golding H. Kinetics of soluble CD4 binding to cells expressing human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1992 Jan;66(1):132–138. doi: 10.1128/jvi.66.1.132-138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. A delay in membrane fusion: lag times observed by fluorescence microscopy of individual fusion events induced by an electric field pulse. Biochemistry. 1990 Sep 11;29(36):8337–8344. doi: 10.1021/bi00488a020. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Willey R. L., Martin M. A., Blumenthal R. Kinetics of HIV-1 interactions with sCD4 and CD4+ cells: implications for inhibition of virus infection and initial steps of virus entry into cells. Virology. 1992 Apr;187(2):398–406. doi: 10.1016/0042-6822(92)90441-q. [DOI] [PubMed] [Google Scholar]
- Dragic T., Charneau P., Clavel F., Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992 Aug;66(8):4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Delwart E. L., Buchschacher G. L., Jr, Panganiban A. T. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe C., Beck A., Gelderblom H. R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3(10):965–974. [PubMed] [Google Scholar]
- Healey D., Dianda L., Moore J. P., McDougal J. S., Moore M. J., Estess P., Buck D., Kwong P. D., Beverley P. C., Sattentau Q. J. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990 Oct 1;172(4):1233–1242. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman K., Shapira-Nahor O., Gruber M. F., Hooley J., Manischewitz J., Seeman R., Vujcic L., Geyer S. J., Golding H. Chemically induced CD4 mutants of a human T cell line. Evidence for dissociation between binding of HIV I envelope and susceptibility to HIV I infection and syncytia formation. J Immunol. 1990 Mar 15;144(6):2131–2139. [PubMed] [Google Scholar]
- König R., Ashwell G., Hanover J. A. Glycosylation of CD4. Tunicamycin inhibits surface expression. J Biol Chem. 1988 Jul 5;263(19):9502–9507. [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Lowy R. J., Sarkar D. P., Chen Y., Blumenthal R. Observation of single influenza virus-cell fusion and measurement by fluorescence video microscopy. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1850–1854. doi: 10.1073/pnas.87.5.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Sattentau Q. J., Klasse P. J., Burkly L. C. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992 Aug;66(8):4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Nair B. C., Hoke G. M., Sarngadharan M. G., Edidin M. Lateral diffusion of CD4 on the surface of a human neoplastic T-cell line probed with a fluorescent derivative of the envelope glycoprotein (gp120) of human immunodeficiency virus type 1 (HIV-1). J Cell Physiol. 1991 May;147(2):326–332. doi: 10.1002/jcp.1041470219. [DOI] [PubMed] [Google Scholar]
- Poncelet P., Carayon P. Cytofluorometric quantification of cell-surface antigens by indirect immunofluorescence using monoclonal antibodies. J Immunol Methods. 1985 Dec 17;85(1):65–74. doi: 10.1016/0022-1759(85)90274-1. [DOI] [PubMed] [Google Scholar]
- Poulin L., Evans L. A., Tang S. B., Barboza A., Legg H., Littman D. R., Levy J. A. Several CD4 domains can play a role in human immunodeficiency virus infection in cells. J Virol. 1991 Sep;65(9):4893–4901. doi: 10.1128/jvi.65.9.4893-4901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D. P., Morris S. J., Eidelman O., Zimmerberg J., Blumenthal R. Initial stages of influenza hemagglutinin-induced cell fusion monitored simultaneously by two fluorescent events: cytoplasmic continuity and lipid mixing. J Cell Biol. 1989 Jul;109(1):113–122. doi: 10.1083/jcb.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Orenstein J., Dimitrov D., Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992 Feb;186(2):712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J. CD4 activation of HIV fusion. Int J Cell Cloning. 1992 Nov;10(6):323–332. doi: 10.1002/stem.5530100603. [DOI] [PubMed] [Google Scholar]
- Simon J. H., Somoza C., Schockmel G. A., Collin M., Davis S. J., Williams A. F., James W. A rat CD4 mutant containing the gp120-binding site mediates human immunodeficiency virus type 1 infection. J Exp Med. 1993 Apr 1;177(4):949–954. doi: 10.1084/jem.177.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]





