Abstract
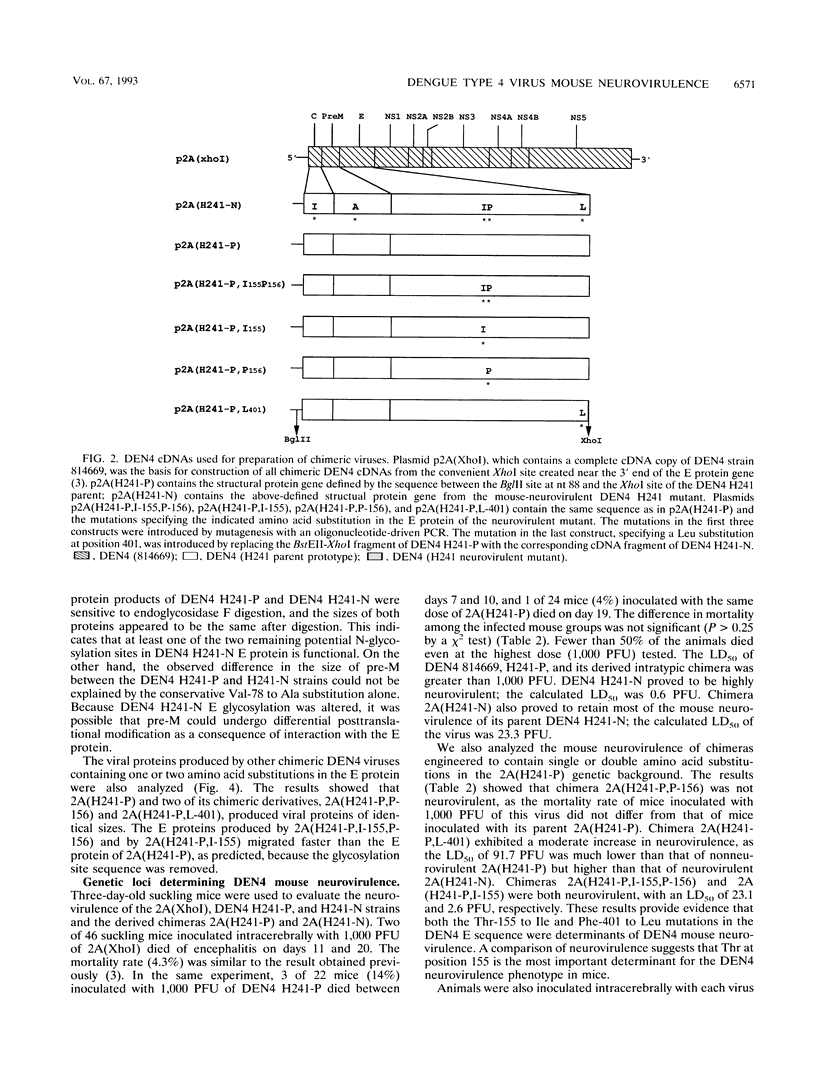
Mouse-adapted dengue type 4 virus (DEN4) strain H241 is highly neurovirulent for mice, whereas its non-mouse-adapted parent is rarely neurovirulent. The genetic basis for the neurovirulence of the mouse-adapted mutant was studied by comparing intratypic chimeric viruses that contained the three structural protein genes from the parental virus or the neurovirulent mutant in the background sequence of nonneurovirulent DEN4 strain 814669. The chimera that contained the three structural protein genes from mouse neurovirulent DEN4 strain H241 proved to be highly neurovirulent in mice, whereas the chimera that contained the corresponding genes from its non-mouse-adapted parent was not neurovirulent. This finding indicates that most of the genetic loci for the neurovirulence of the DEN4 mutant lie within the structural protein genes. A comparison of the amino acid sequences of the parent and its mouse neurovirulent mutant proteins revealed that there were only five amino acid differences in the structural protein region, and three of these were located in the envelope (E) glycoprotein. Analysis of chimeras which contained one or two of the variant amino acids of the mutant E sequence substituting for the corresponding sequence of the parental virus identified two of these amino acid changes as important determinants of mouse neurovirulence. First, the single substitution of Ile for Thr-155 which ablated one of the two conserved glycosylation sites in parental E yielded a virus that was almost as neurovirulent as the mouse-adapted mutant. Thus, the loss of an E glycosylation site appears to play a role in DEN4 neurovirulence. Second, the substitution of Leu for Phe-401 also yielded a neurovirulent virus, but it was less neurovirulent than the glycosylation mutant. These findings indicate that at least two of the genetic loci responsible for DEN4 mouse neurovirulence map within the structural protein genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok J., Samuel S., Gibbs A. J., Vitarana U. T. Variation of the nucleotide and encoded amino acid sequences of the envelope gene from eight dengue-2 viruses. Arch Virol. 1989;105(1-2):39–53. doi: 10.1007/BF01311115. [DOI] [PubMed] [Google Scholar]
- Bray M., Lai C. J. Construction of intertypic chimeric dengue viruses by substitution of structural protein genes. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10342–10346. doi: 10.1073/pnas.88.22.10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Zhao B. T., Markoff L., Eckels K. H., Chanock R. M., Lai C. J. Mice immunized with recombinant vaccinia virus expressing dengue 4 virus structural proteins with or without nonstructural protein NS1 are protected against fatal dengue virus encephalitis. J Virol. 1989 Jun;63(6):2853–2856. doi: 10.1128/jvi.63.6.2853-2856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coia G., Parker M. D., Speight G., Byrne M. E., Westaway E. G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988 Jan;69(Pt 1):1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Trent D. W., Strauss J. H., Rice C. M. Partial nucleotide sequence of the Murray Valley encephalitis virus genome. Comparison of the encoded polypeptides with yellow fever virus structural and non-structural proteins. J Mol Biol. 1986 Feb 5;187(3):309–323. doi: 10.1016/0022-2836(86)90435-3. [DOI] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Trent D. W. Nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue type 2 virus, Jamaica genotype. Virology. 1986 Dec;155(2):365–377. doi: 10.1016/0042-6822(86)90200-x. [DOI] [PubMed] [Google Scholar]
- Deubel V., Schlesinger J. J., Digoutte J. P., Girard M. Comparative immunochemical and biological analysis of African and South American yellow fever viruses. Arch Virol. 1987;94(3-4):331–338. doi: 10.1007/BF01310727. [DOI] [PubMed] [Google Scholar]
- Gruenberg A., Woo W. S., Biedrzycka A., Wright P. J. Partial nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue virus type 2, New Guinea C and PUO-218 strains. J Gen Virol. 1988 Jun;69(Pt 6):1391–1398. doi: 10.1099/0022-1317-69-6-1391. [DOI] [PubMed] [Google Scholar]
- HAMMON W. M., RUDNICK A., SATHER G. E. Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science. 1960 Apr 15;131(3407):1102–1103. doi: 10.1126/science.131.3407.1102. [DOI] [PubMed] [Google Scholar]
- HAMMON W. M., RUDNICK A., SATHER G., ROGERS K. D., MORSE L. J. New hemorrhagic fevers of children in the Philippines and Thailand. Trans Assoc Am Physicians. 1960;73:140–155. [PubMed] [Google Scholar]
- HOTTA S. Experimental studies on dengue. I. Isolation, identification and modification of the virus. J Infect Dis. 1952 Jan-Feb;90(1):1–9. doi: 10.1093/infdis/90.1.1. [DOI] [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Henchal E. A., Repik P. M., McCown J. M., Brandt W. E. Identification of an antigenic and genetic variant of dengue-4 virus from the Caribbean. Am J Trop Med Hyg. 1986 Mar;35(2):393–400. doi: 10.4269/ajtmh.1986.35.393. [DOI] [PubMed] [Google Scholar]
- Irie K., Mohan P. M., Sasaguri Y., Putnak R., Padmanabhan R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain). Gene. 1989 Feb 20;75(2):197–211. doi: 10.1016/0378-1119(89)90266-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Zhao B. T., Hori H., Bray M. Infectious RNA transcribed from stably cloned full-length cDNA of dengue type 4 virus. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5139–5143. doi: 10.1073/pnas.88.12.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., McAda P. C., Mason T. L., Fournier M. J. Sequence of the dengue-1 virus genome in the region encoding the three structural proteins and the major nonstructural protein NS1. Virology. 1987 Nov;161(1):262–267. doi: 10.1016/0042-6822(87)90196-6. [DOI] [PubMed] [Google Scholar]
- Men R. H., Bray M., Lai C. J. Carboxy-terminally truncated dengue virus envelope glycoproteins expressed on the cell surface and secreted extracellularly exhibit increased immunogenicity in mice. J Virol. 1991 Mar;65(3):1400–1407. doi: 10.1128/jvi.65.3.1400-1407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osatomi K., Sumiyoshi H. Complete nucleotide sequence of dengue type 3 virus genome RNA. Virology. 1990 Jun;176(2):643–647. doi: 10.1016/0042-6822(90)90037-r. [DOI] [PubMed] [Google Scholar]
- Pletnev A. G., Bray M., Huggins J., Lai C. J. Construction and characterization of chimeric tick-borne encephalitis/dengue type 4 viruses. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10532–10536. doi: 10.1073/pnas.89.21.10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post P. R., Santos C. N., Carvalho R., Cruz A. C., Rice C. M., Galler R. Heterogeneity in envelope protein sequence and N-linked glycosylation among yellow fever virus vaccine strains. Virology. 1992 May;188(1):160–167. doi: 10.1016/0042-6822(92)90745-b. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Noncytopathogenic variants of poliomyelitis viruses and resistance to superinfection in tissue culture. Science. 1954 Aug 27;120(3113):357–357. doi: 10.1126/science.120.3113.357. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Recent advances in our knowledge of dengue and sandfly fever. Am J Trop Med Hyg. 1955 Mar;4(2):198–207. doi: 10.4269/ajtmh.1955.4.198. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Research on dengue during World War II. Am J Trop Med Hyg. 1952 Jan;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER W., FRANKEL J. W. Adaptation of the New Guinea B strain of dengue virus to suckling and to adult swiss mice; a study in viral variation. Am J Trop Med Hyg. 1952 Jan;1(1):66–77. doi: 10.4269/ajtmh.1952.1.66. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Schlesinger R. W. PRODUCTION OF IMMUNITY TO DENGUE WITH VIRUS MODIFIED BY PROPAGATION IN MICE. Science. 1945 Jun 22;101(2634):640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- Wengler G., Castle E., Leidner U., Nowak T., Wengler G. Sequence analysis of the membrane protein V3 of the flavivirus West Nile virus and of its gene. Virology. 1985 Dec;147(2):264–274. doi: 10.1016/0042-6822(85)90129-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Hayes E. P., McCarty T. C., Dubois D. R., Summers P. L., Eckels K. H., Chanock R. M., Lai C. J. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induces resistance to dengue virus encephalitis. J Virol. 1988 Aug;62(8):3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Mackow E., Buckler-White A., Markoff L., Chanock R. M., Lai C. J., Makino Y. Cloning full-length dengue type 4 viral DNA sequences: analysis of genes coding for structural proteins. Virology. 1986 Nov;155(1):77–88. doi: 10.1016/0042-6822(86)90169-8. [DOI] [PubMed] [Google Scholar]





