Abstract
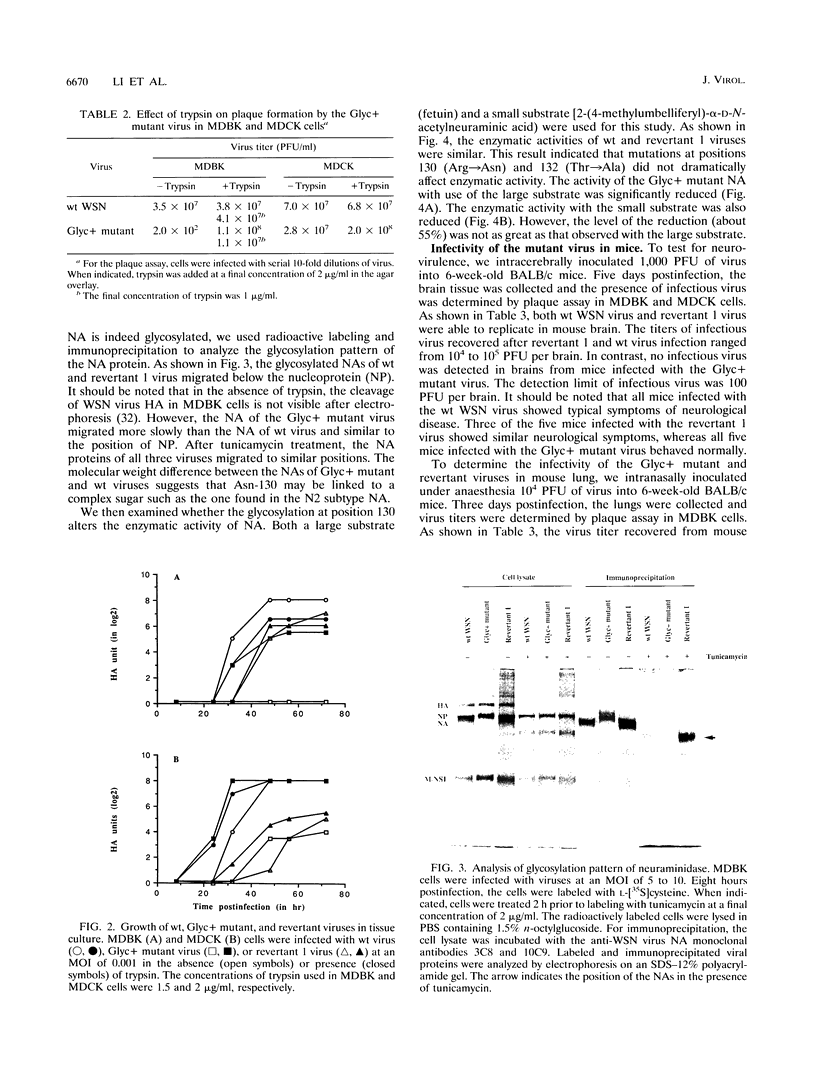
The neuraminidase (NA) gene of influenza A/WSN/33 (WSN) virus has previously been shown to be associated with neurovirulence in mice and growth in Madin-Darby bovine kidney (MDBK) cells. Nucleotide sequence analysis has indicated that the NA of WSN virus lacks a conserved glycosylation site at position 130 (corresponding to position 146 in the N2 subtype). To investigate the role of this carbohydrate in viral pathogenicity, we used reverse genetics methods to generate a Glyc+ mutant virus, in which the glycosylation site Asn-130 was introduced into the WSN virus NA. Unlike the wild-type WSN virus, the Glyc+ mutant virus did not undergo multicycle replication in MDBK cells in the absence of trypsin, presumably because of lack of cleavage activation of infectivity. In contrast, revertant viruses derived from the Glyc+ mutant were able to replicate in MDBK cells without exogenous protease. Nucleotide sequence analysis revealed that the NAs of the revertant viruses had lost the introduced glycosylation site. In contrast to wild-type and revertant viruses, the Glyc+ mutant virus was not able to multiply in mouse brain. These results suggest that the absence of a glycosylation site at position 130 of the NA plays a key role in the neurovirulence of WSN virus in mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Air G. M., Laver W. G. The neuraminidase of influenza virus. Proteins. 1989;6(4):341–356. doi: 10.1002/prot.340060402. [DOI] [PubMed] [Google Scholar]
- Baker A. T., Varghese J. N., Laver W. G., Air G. M., Colman P. M. Three-dimensional structure of neuraminidase of subtype N9 from an avian influenza virus. Proteins. 1987;2(2):111–117. doi: 10.1002/prot.340020205. [DOI] [PubMed] [Google Scholar]
- Basak S., Pritchard D. G., Bhown A. S., Compans R. W. Glycosylation sites of influenza viral glycoproteins: characterization of tryptic glycopeptides from the A/USSR(H1N1) hemagglutinin glycoprotein. J Virol. 1981 Feb;37(2):549–558. doi: 10.1128/jvi.37.2.549-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S., Tomana M., Compans R. W. Sialic acid is incorporated into influenza hemagglutinin glycoproteins in the absence of viral neuraminidase. Virus Res. 1985 Feb;2(1):61–68. doi: 10.1016/0168-1702(85)90060-7. [DOI] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P., Ruigrok R. W., Cusack S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992 Jan;11(1):49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci M. R., Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J Virol. 1993 Feb;67(2):759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Ward C. W. Structure and diversity of influenza virus neuraminidase. Curr Top Microbiol Immunol. 1985;114:177–255. doi: 10.1007/978-3-642-70227-3_5. [DOI] [PubMed] [Google Scholar]
- Enami M., Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991 May;65(5):2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Naeve C. W., Webster R. G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984 Dec;139(2):303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Keil W., Geyer R., Dabrowski J., Dabrowski U., Niemann H., Stirm S., Klenk H. D. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J. 1985 Oct;4(10):2711–2720. doi: 10.1002/j.1460-2075.1985.tb03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz M. R., Webster R. G., Air G. M. Site-directed mutation of the active site of influenza neuraminidase and implications for the catalytic mechanism. Biochemistry. 1987 Aug 25;26(17):5351–5358. doi: 10.1021/bi00391a020. [DOI] [PubMed] [Google Scholar]
- Li S. Q., Schulman J. L., Moran T., Bona C., Palese P. Influenza A virus transfectants with chimeric hemagglutinins containing epitopes from different subtypes. J Virol. 1992 Jan;66(1):399–404. doi: 10.1128/jvi.66.1.399-404.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Air G. M. Selection and characterization of a neuraminidase-minus mutant of influenza virus and its rescue by cloned neuraminidase genes. Virology. 1993 May;194(1):403–407. doi: 10.1006/viro.1993.1276. [DOI] [PubMed] [Google Scholar]
- Luo G., Chung J., Palese P. Alterations of the stalk of the influenza virus neuraminidase: deletions and insertions. Virus Res. 1993 Aug;29(2):141–153. doi: 10.1016/0168-1702(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Mayer V., Schulman J. L., Kilbourne E. D. Nonlinkage of neurovirulence exclusively to viral hemagglutinin or neuraminidase in genetic recombinants of A-NWS (HON1) influenza virus. J Virol. 1973 Feb;11(2):272–278. doi: 10.1128/jvi.11.2.272-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T., Subbarao E. K., Enami M., Murphy B. R., Palese P. An influenza A virus containing influenza B virus 5' and 3' noncoding regions on the neuraminidase gene is attenuated in mice. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5177–5181. doi: 10.1073/pnas.88.12.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. W., Lee R. T., Lee Y. C., Thomas G. H., Reynolds L. W., Uchida Y. The synthesis of 4-methylumbelliferyl alpha-ketoside of N-acetylneuraminic acid and its use in a fluorometric assay for neuraminidase. Anal Biochem. 1980 Jan 1;101(1):166–174. doi: 10.1016/0003-2697(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Sugiura A. Neurovirulence of influenza virus in mice. II. Mechanism of virulence as studied in a neuroblastoma cell line. Virology. 1980 Mar;101(2):450–457. doi: 10.1016/0042-6822(80)90458-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Vallbracht A., Flehmig B., Rott R. Correlation of pathogenicity and gene constellation of influenza A viruses. II. Highly neurovirulent recombinants derived from non-neurovirulent or weakly neurovirulent parent virus strains. Virology. 1979 Jun;95(2):492–500. doi: 10.1016/0042-6822(79)90503-8. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977 Oct;24(1):170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J. T., Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966 Dec;30(4):731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- Sugiura A., Ueda M. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology. 1980 Mar;101(2):440–449. doi: 10.1016/0042-6822(80)90457-2. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Elleman T. C., Azad A. A. Amino acid sequence of the Pronase-released heads of neuraminidase subtype N2 from the Asian strain A/Tokyo/3/67 of influenza virus. Biochem J. 1982 Oct 1;207(1):91–95. doi: 10.1042/bj2070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. W., Murray J. M., Roxburgh C. M., Jackson D. C. Chemical and antigenic characterization of the carbohydrate side chains of an Asian (N2) influenza virus neuraminidase. Virology. 1983 Apr 15;126(1):370–375. doi: 10.1016/0042-6822(83)90486-5. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol. 1967 Jul;99(1):49–55. [PubMed] [Google Scholar]