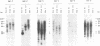Abstract
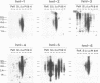
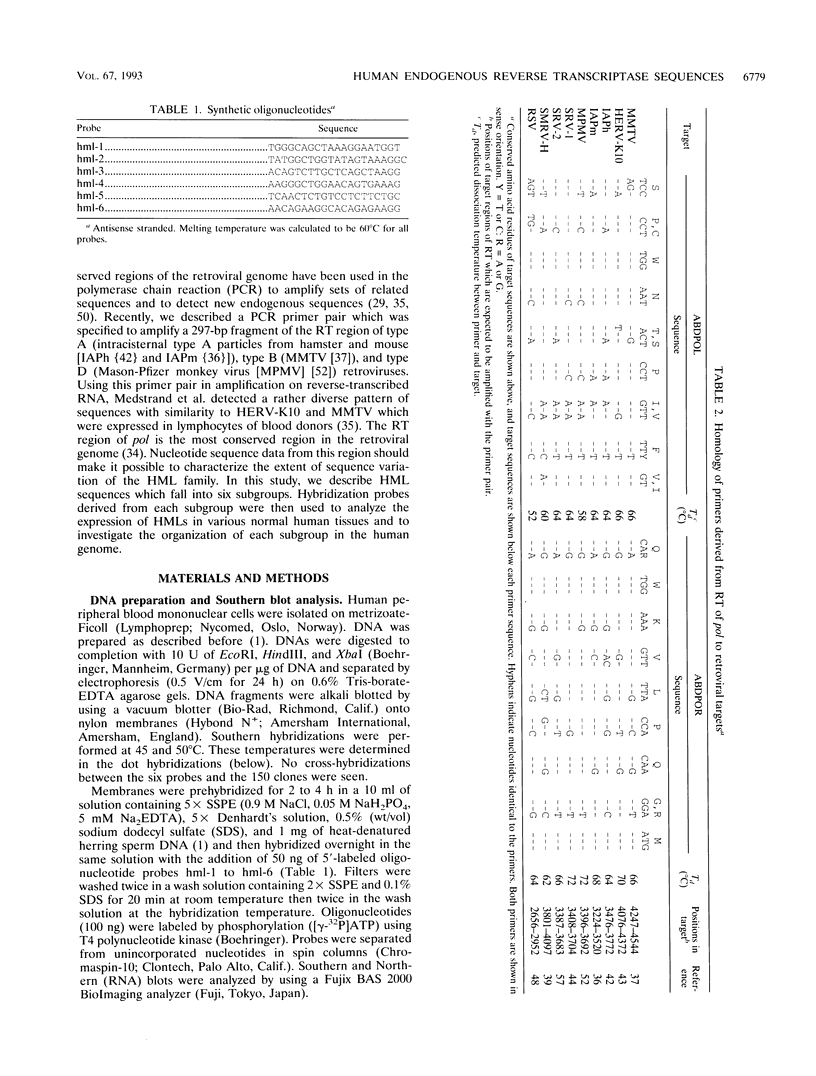
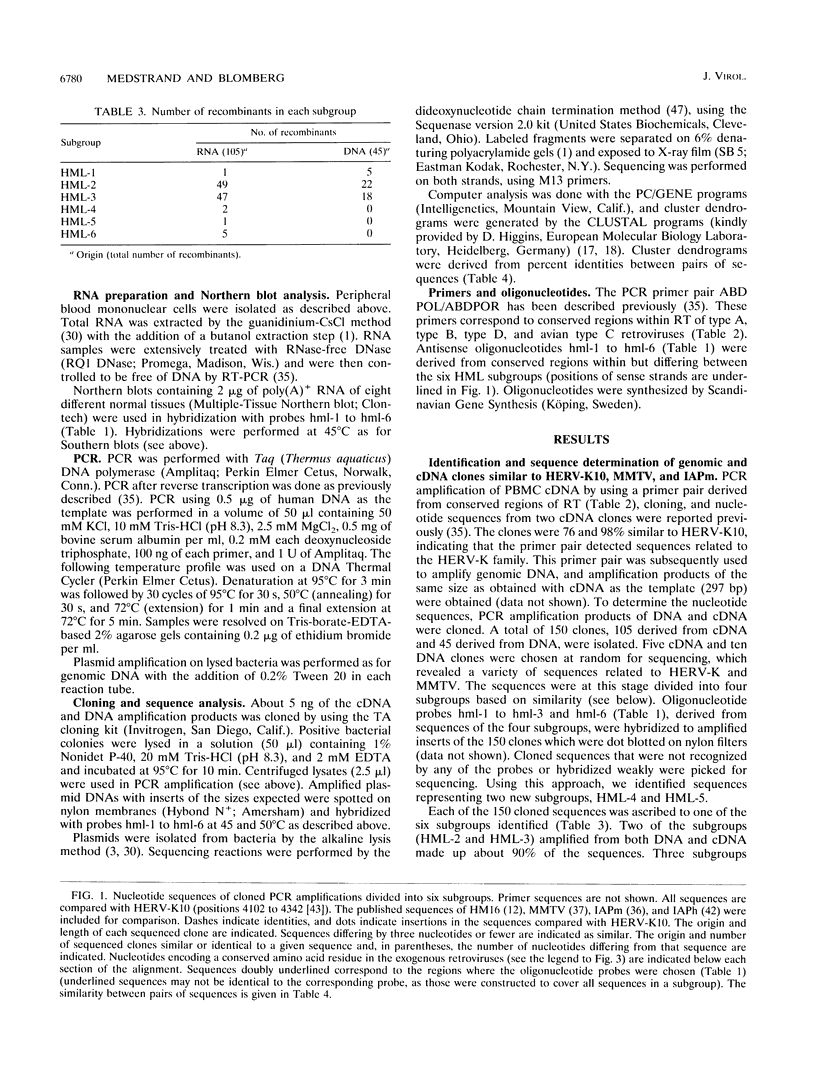
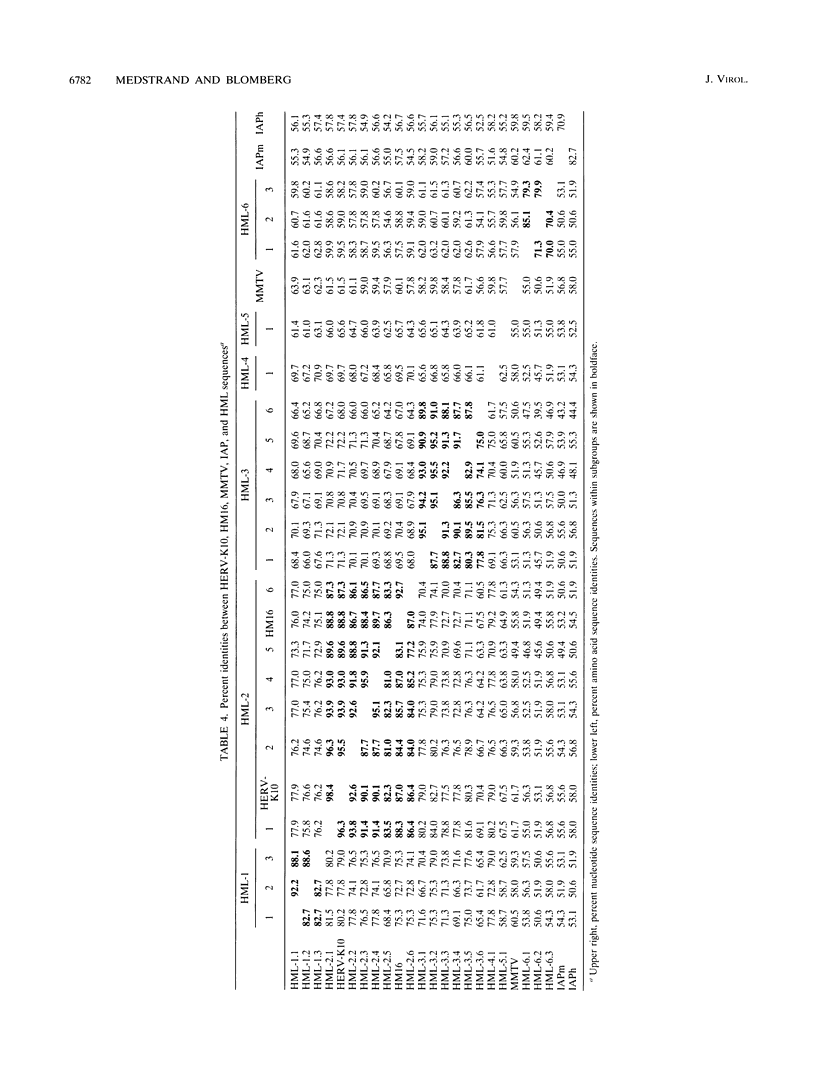
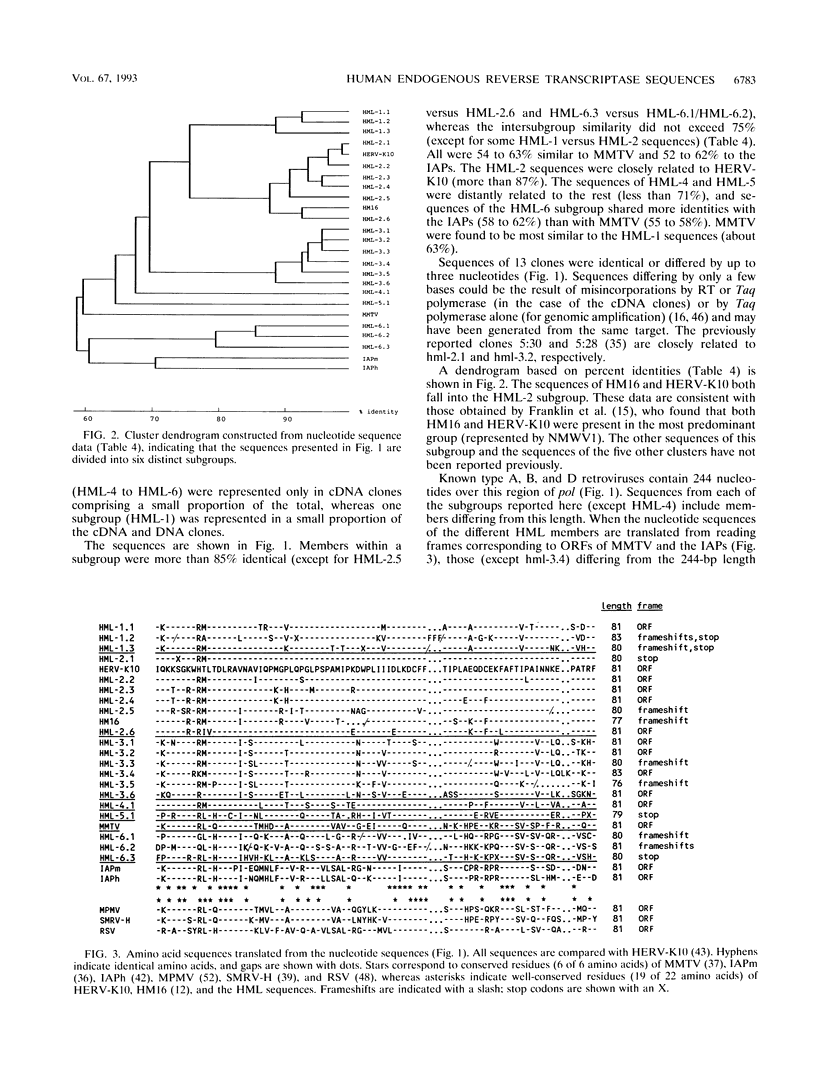
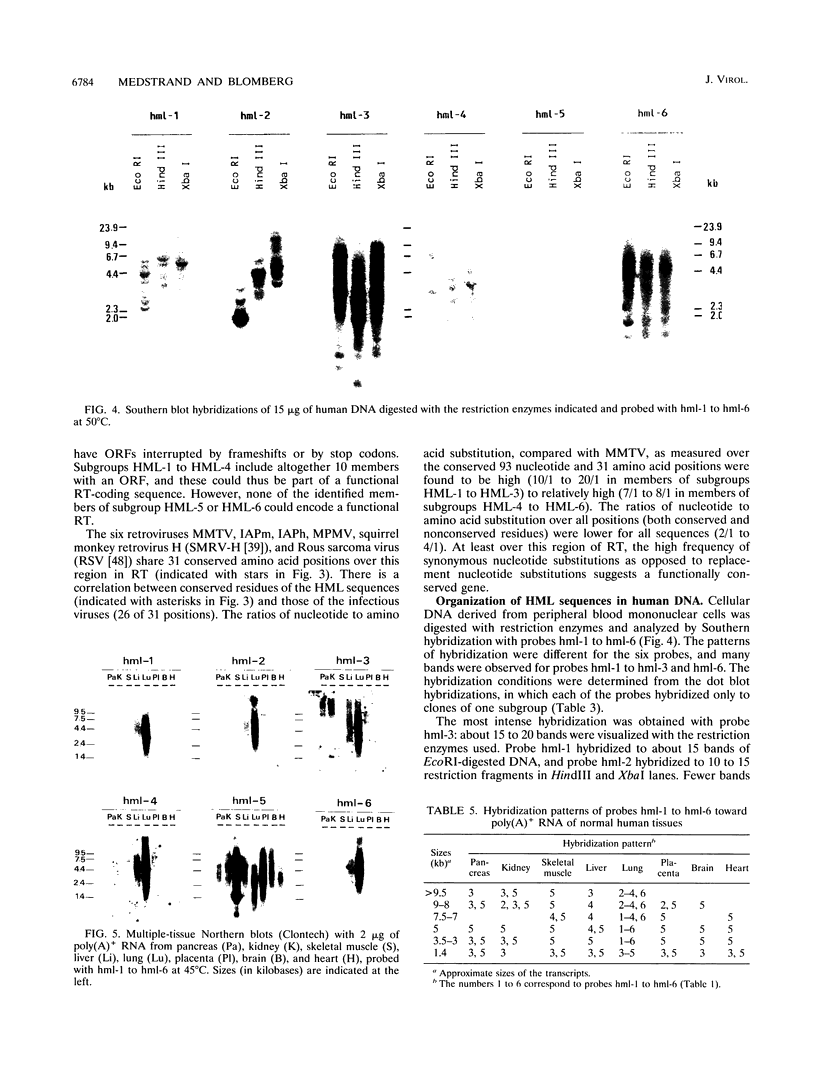
The polymerase chain reaction was used to amplify genomic DNA and reverse-transcribed RNA from human lymphocytes, using primers derived from conserved regions within the retroviral reverse transcriptase. Sequencing of 33 cloned amplification products revealed that a variety of sequences with similarity to mouse mammary tumor virus, mouse intracisternal A particle, and human endogenous retrovirus K10 were detected with this primer pair. The sequences were divided into six subgroups, with a nucleotide sequence dissimilarity of about 25% between the subgroups. Members within five of the subgroups were most closely related to human endogenous retrovirus K10 and mouse mammary tumor virus, whereas sequences of the sixth subgroup also showed similarity to mouse intracisternal A particle. Ten of the sequences had open reading frames with preference for silent mutations at conserved sites. Southern blot analysis showed that some HML (human endogenous MMTV-like) subgroups (HML-4 and HML-5) were present in a few copies (about 5), whereas others (HML-1 to HML-3 and HML-6) were present in at least 10 to 20 copies per genome. Northern (RNA) blot analysis revealed that several of the subgroups are differentially expressed in human normal tissues. A complex pattern of transcripts from about 12 to 1.4 kb was found in several of the tissues tested. However, the most abundant expression was detected in lung (all subgroups), skeletal muscle (HML-4 and HML-5), placenta (HML-2 and HML-5), and kidney (HML-2, HML-3 and HML-5). Expression of reverse transcriptase sequences in human tissues may have biological consequences. The described sequences are similar to elements which cause carcinoma and are immunoregulatory in mice. It remains to be seen whether human sequences also have such functions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R., Leib-Mösch C., Werner T., Erfle V., Hehlmann R. Human endogenous retrovirus-like sequences. Haematol Blood Transfus. 1989;32:464–477. doi: 10.1007/978-3-642-74621-5_81. [DOI] [PubMed] [Google Scholar]
- Brodsky I., Foley B., Haines D., Johnston J., Cuddy K., Gillespie D. Expression of HERV-K proviruses in human leukocytes. Blood. 1993 May 1;81(9):2369–2374. [PubMed] [Google Scholar]
- Callahan R., Chiu I. M., Wong J. F., Tronick S. R., Roe B. A., Aaronson S. A., Schlom J. A new class of endogenous human retroviral genomes. Science. 1985 Jun 7;228(4704):1208–1211. doi: 10.1126/science.2408338. [DOI] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Tronick S., Schlom J. Detection and cloning of human DNA sequences related to the mouse mammary tumor virus genome. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5503–5507. doi: 10.1073/pnas.79.18.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen K. C., Sweet R. W. Murine mammary tumor virus pol-related sequences in human DNA: characterization and sequence comparison with the complete murine mammary tumor virus pol gene. J Virol. 1986 Feb;57(2):422–432. doi: 10.1128/jvi.57.2.422-432.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou-Pachnis A., Lohse M. A., Furano A. V., Tsichlis P. N. Insertion of long interspersed repeated elements at the Igh (immunoglobulin heavy chain) and Mlvi-2 (Moloney leukemia virus integration 2) loci of rats. Proc Natl Acad Sci U S A. 1985 May;82(9):2857–2861. doi: 10.1073/pnas.82.9.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J. Transposable elements in eukaryotes. Int Rev Cytol. 1985;93:281–326. doi: 10.1016/s0074-7696(08)61376-5. [DOI] [PubMed] [Google Scholar]
- Franklin G. C., Chretien S., Hanson I. M., Rochefort H., May F. E., Westley B. R. Expression of human sequences related to those of mouse mammary tumor virus. J Virol. 1988 Apr;62(4):1203–1210. doi: 10.1128/jvi.62.4.1203-1210.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirose Y., Takamatsu M., Harada F. Presence of env genes in members of the RTVL-H family of human endogenous retrovirus-like elements. Virology. 1993 Jan;192(1):52–61. doi: 10.1006/viro.1993.1007. [DOI] [PubMed] [Google Scholar]
- Horn T. M., Huebner K., Croce C., Callahan R. Chromosomal locations of members of a family of novel endogenous human retroviral genomes. J Virol. 1986 Jun;58(3):955–959. doi: 10.1128/jvi.58.3.955-959.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Pfeifer-Ohlsson S., Kato M., Larsson E., Rydnert J., Ohlsson R., Cohen M. Tissue-specific expression of human provirus ERV3 mRNA in human placenta: two of the three ERV3 mRNAs contain human cellular sequences. J Virol. 1987 Jul;61(7):2182–2191. doi: 10.1128/jvi.61.7.2182-2191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Klinman D. M., Perl A., Steinberg A. D. Heterogeneous expression and coordinate regulation of endogenous retroviral sequences in human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1992 Dec;8(12):1991–1998. doi: 10.1089/aid.1992.8.1991. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Perl A. Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J. 1992 May;6(8):2537–2544. doi: 10.1096/fasebj.6.8.1592206. [DOI] [PubMed] [Google Scholar]
- La Mantia G., Maglione D., Pengue G., Di Cristofano A., Simeone A., Lanfrancone L., Lania L. Identification and characterization of novel human endogenous retroviral sequences prefentially expressed in undifferentiated embryonal carcinoma cells. Nucleic Acids Res. 1991 Apr 11;19(7):1513–1520. doi: 10.1093/nar/19.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania L., Di Cristofano A., Strazzullo M., Pengue G., Majello B., La Mantia G. Structural and functional organization of the human endogenous retroviral ERV9 sequences. Virology. 1992 Nov;191(1):464–468. doi: 10.1016/0042-6822(92)90211-7. [DOI] [PubMed] [Google Scholar]
- Larsson E., Kato N., Cohen M. Human endogenous proviruses. Curr Top Microbiol Immunol. 1989;148:115–132. doi: 10.1007/978-3-642-74700-7_4. [DOI] [PubMed] [Google Scholar]
- Lindeskog M., Medstrand P., Blomberg J. Sequence variation of human endogenous retrovirus ERV9-related elements in an env region corresponding to an immunosuppressive peptide: transcription in normal and neoplastic cells. J Virol. 1993 Feb;67(2):1122–1126. doi: 10.1128/jvi.67.2.1122-1126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani-Costantini R., Horn T. M., Callahan R. Ancestry of a human endogenous retrovirus family. J Virol. 1989 Nov;63(11):4982–4985. doi: 10.1128/jvi.63.11.4982-4985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R., Rochefort H., Buetti E., Diggelmann H. Mouse mammary tumour virus related sequences are present in human DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4127–4139. doi: 10.1093/nar/11.12.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Structure of a human retroviral sequence related to mouse mammary tumor virus. J Virol. 1986 Nov;60(2):743–749. doi: 10.1128/jvi.60.2.743-749.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Feng D. F., Doolittle R. F. Sequence comparisons of retroviral proteins: relative rates of change and general phylogeny. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2469–2473. doi: 10.1073/pnas.85.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medstrand P., Lindeskog M., Blomberg J. Expression of human endogenous retroviral sequences in peripheral blood mononuclear cells of healthy individuals. J Gen Virol. 1992 Sep;73(Pt 9):2463–2466. doi: 10.1099/0022-1317-73-9-2463. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse B., Rotherg P. G., South V. J., Spandorfer J. M., Astrin S. M. Insertional mutagenesis of the myc locus by a LINE-1 sequence in a human breast carcinoma. Nature. 1988 May 5;333(6168):87–90. doi: 10.1038/333087a0. [DOI] [PubMed] [Google Scholar]
- Oda T., Ikeda S., Watanabe S., Hatsushika M., Akiyama K., Mitsunobu F. Molecular cloning, complete nucleotide sequence, and gene structure of the provirus genome of a retrovirus produced in a human lymphoblastoid cell line. Virology. 1988 Dec;167(2):468–476. [PubMed] [Google Scholar]
- Ono M., Kawakami M., Ushikubo H. Stimulation of expression of the human endogenous retrovirus genome by female steroid hormones in human breast cancer cell line T47D. J Virol. 1987 Jun;61(6):2059–2062. doi: 10.1128/jvi.61.6.2059-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Molecular cloning and long terminal repeat sequences of human endogenous retrovirus genes related to types A and B retrovirus genes. J Virol. 1986 Jun;58(3):937–944. doi: 10.1128/jvi.58.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shih A., Coutavas E. E., Rush M. G. Evolutionary implications of primate endogenous retroviruses. Virology. 1991 Jun;182(2):495–502. doi: 10.1016/0042-6822(91)90590-8. [DOI] [PubMed] [Google Scholar]
- Shih A., Misra R., Rush M. G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989 Jan;63(1):64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Martin M. A., Rabson A. B., Bryan T., O'Brien S. J. Amplification and chromosomal dispersion of human endogenous retroviral sequences. J Virol. 1986 Sep;59(3):545–550. doi: 10.1128/jvi.59.3.545-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Reverse transcription in the eukaryotic genome: retroviruses, pararetroviruses, retrotransposons, and retrotranscripts. Mol Biol Evol. 1985 Nov;2(6):455–468. doi: 10.1093/oxfordjournals.molbev.a040365. [DOI] [PubMed] [Google Scholar]
- Thayer R. M., Power M. D., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Sequence relationships of type D retroviruses which cause simian acquired immunodeficiency syndrome. Virology. 1987 Apr;157(2):317–329. doi: 10.1016/0042-6822(87)90274-1. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Westley B., May F. E. The human genome contains multiple sequences of varying homology to mouse mammary tumour virus DNA. Gene. 1984 May;28(2):221–227. doi: 10.1016/0378-1119(84)90259-2. [DOI] [PubMed] [Google Scholar]