Abstract
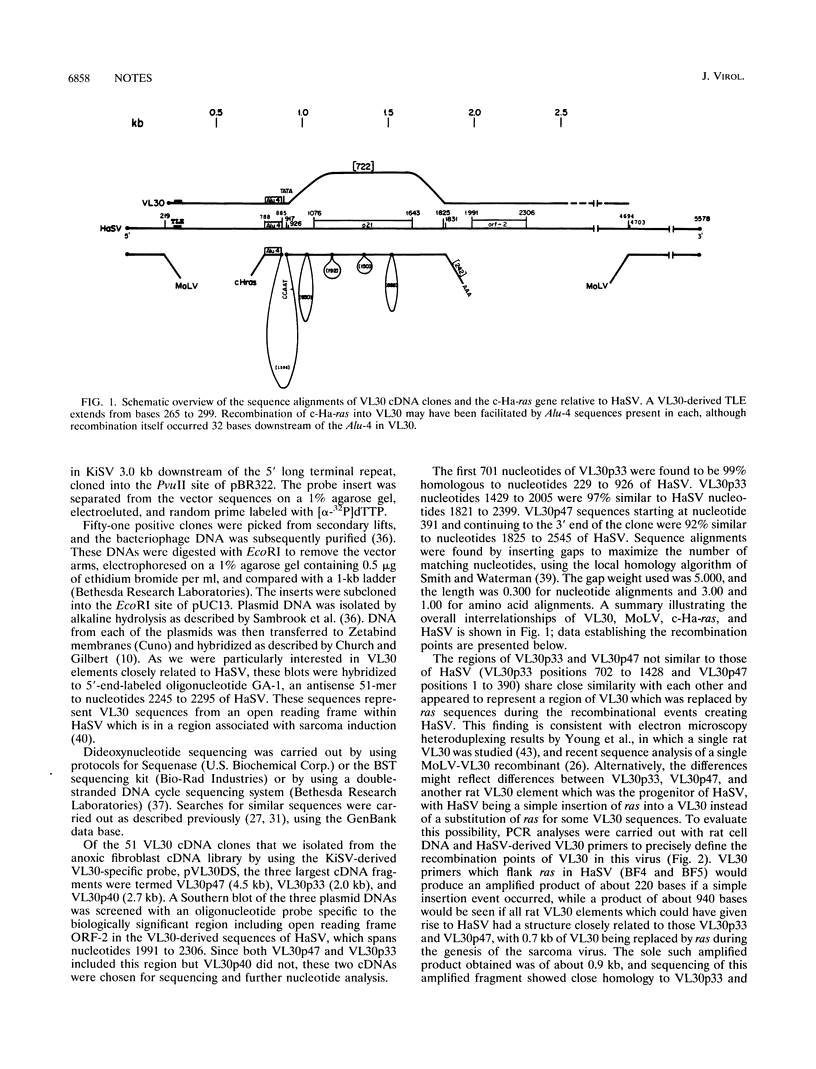
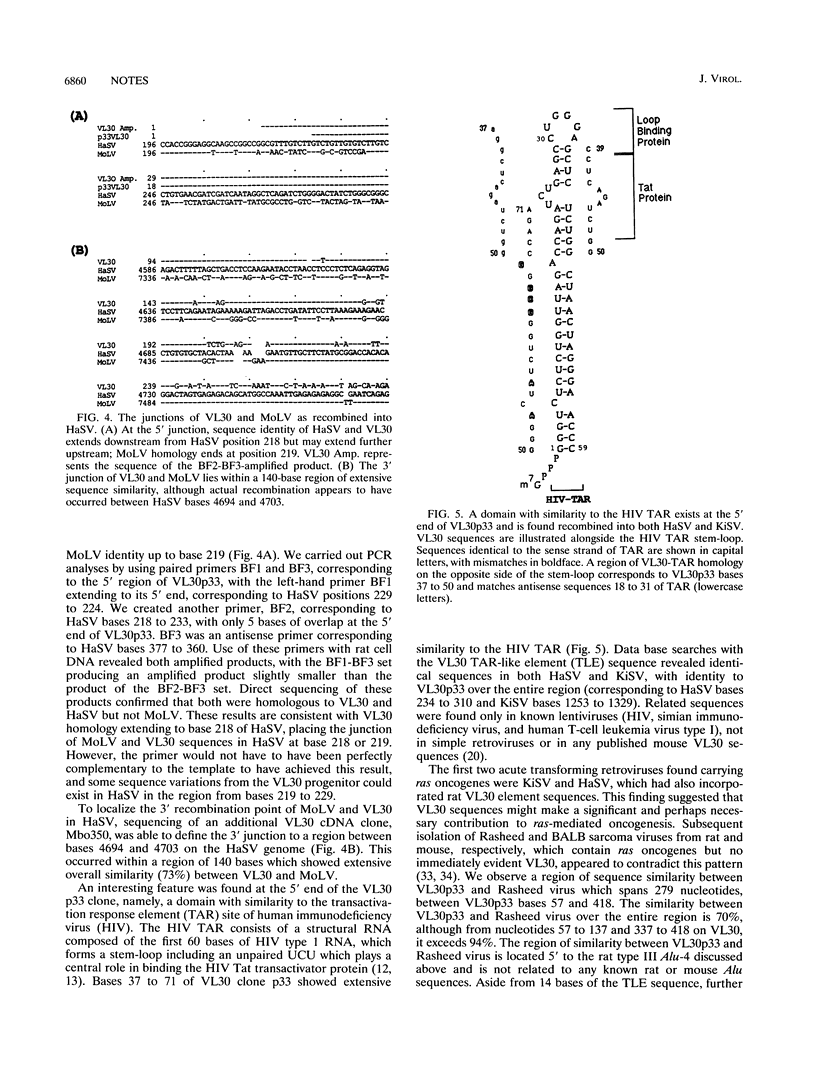
VL30 elements are associated with cancer by their overexpression in rodent malignancies, their induction in a fibroblast response to anoxia which shares features with the malignant phenotype, and their presence recombined into Harvey murine sarcoma virus (HaSV) and Kirsten murine sarcoma virus. These sarcoma viruses contain ras oncogenes flanked on both sides by retrotransposon VL30 element sequences, in turn flanked by mouse leukemia virus sequences. Three very basic questions have existed about the VL30 element sequences found in sarcoma viruses: (i) how did they become recombined, (ii) what are their exact boundaries, and (iii) why are they there? To help decipher the nature of VL30 elements in sarcoma viruses, we examined VL30 clones isolated from an anoxic fibroblast cDNA library and independently by polymerase chain reaction cloning from rat cell DNA. Sequence comparisons with HaSV revealed that HaSV was formed by the substitution of 0.7 kb of VL30 sequences by 0.9 kb of c-Ha-ras sequences, with this event possibly facilitated by the presence of an identical Alu-like repeat found upstream of the 5' recombination point in both the VL30 element and c-Ha-ras. Recombination occurred 42 bases beyond the Alu-like sequences in VL30 and 1596 bases beyond them in c-Ha-ras, at position 926 of HaSV. The 3' ras-VL30 recombination event in HaSV occurred within a seven-base region of shared sequence identity, between HaSV bases 1825 and 1825 and 1831. Recombination between Moloney leukemia virus (MoLV) and VL30 appears to have occurred at a point corresponding to base 218 or 219 of MoLV and was near a TAR-like VL30 sequence; such recombination at the 3' end was between positions 7445 and 7456 of MoLV (HaSV positions 4694 to 4703). Kirsten murine sarcoma virus was found to be closely analogous to HaSV, and limited similar features were also seen with Rasheed sarcoma virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Rathjen P. D., Stanway C. A., Fulton S. M., Malim M. H., Wilson W., Ogden J., King L., Kingsman S. M., Kingsman A. J. Complete nucleotide sequence of a mouse VL30 retro-element. Mol Cell Biol. 1988 Aug;8(8):2989–2998. doi: 10.1128/mcb.8.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. R., Matovcik L. M. Expression of murine sarcoma virus genes in uninfected rat cells subjected to anaerobic sress. Science. 1977 Sep 30;197(4311):1371–1374. doi: 10.1126/science.197602. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Robbins K. C. Rat sequences of the Kirsten and Harvey murine sarcoma virus genomes: nature, origin, and expression in rat tumor RNA. J Virol. 1976 Feb;17(2):335–351. doi: 10.1128/jvi.17.2.335-351.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L. Anoxia, wound healing, VL30 elements, and the molecular basis of malignant conversion. Bioessays. 1993 Apr;15(4):265–272. doi: 10.1002/bies.950150407. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L., Scarcello L. A. Normal fibroblasts responding to anoxia exhibit features of the malignant phenotype. J Biol Chem. 1989 Sep 5;264(25):14885–14892. [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L., Scarcello L. A. Retrotransposon-like VL30 elements are efficiently induced in anoxic rat fibroblasts. J Mol Biol. 1989 Feb 20;205(4):765–769. doi: 10.1016/0022-2836(89)90320-3. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Bouchard L., Lamarre L., Tremblay P. J., Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989 Jun 16;57(6):931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Cichutek K., Duesberg P. H. Transforming function of proto-ras genes depends on heterologous promoters and is enhanced by specific point mutations. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2217–2221. doi: 10.1073/pnas.88.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. A., Garcia-Blanco M. A. Unusual structure of the human immunodeficiency virus type 1 trans-activation response element. J Virol. 1992 Feb;66(2):930–935. doi: 10.1128/jvi.66.2.930-935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of human immunodeficiency virus replication. Annu Rev Microbiol. 1991;45:219–250. doi: 10.1146/annurev.mi.45.100191.001251. [DOI] [PubMed] [Google Scholar]
- Dragani T. A., Manenti G., Della Porta G., Gattoni-Celli S., Weinstein I. B. Expression of retroviral sequences and oncogenes in murine hepatocellular tumors. Cancer Res. 1986 Apr;46(4 Pt 2):1915–1919. [PubMed] [Google Scholar]
- Ecker D. J., Vickers T. A., Bruice T. W., Freier S. M., Jenison R. D., Manoharan M., Zounes M. Pseudo--half-knot formation with RNA. Science. 1992 Aug 14;257(5072):958–961. doi: 10.1126/science.1502560. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Friel J., Hughes D., Pragnell I., Stocking C., Laker C., Nowock J., Ostertag W., Padua R. A. The malignant histiocytosis sarcoma virus, a recombinant of Harvey murine sarcoma virus and Friend mink cell focus-forming virus, has acquired myeloid transformation specificity by alterations in the long terminal repeat. J Virol. 1990 Jan;64(1):369–378. doi: 10.1128/jvi.64.1.369-378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J Virol. 1983 Sep;47(3):478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Han K. A., Rothberg P., Kulesz-Martin M. Altered levels of endogenous retrovirus-like sequence (VL30) RNA during mouse epidermal cell carcinogenesis. Mol Carcinog. 1990;3(2):75–82. doi: 10.1002/mc.2940030205. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Garrett E. D., Cullen B. R. The Bel-1 protein of human foamy virus activates human immunodeficiency virus type 1 gene expression via a novel DNA target site. J Virol. 1992 Jun;66(6):3946–3949. doi: 10.1128/jvi.66.6.3946-3949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Makris A., Patriotis C., Bear S. E., Tsichlis P. N. Structure of a Moloney murine leukemia virus-virus-like 30 recombinant: implications for transduction of the c-Ha-ras proto-oncogene. J Virol. 1993 Mar;67(3):1286–1291. doi: 10.1128/jvi.67.3.1286-1291.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Anderson G. R., Stoler D. L. Harvey sarcoma virus genome contains no extensive sequences unrelated to those of other retroviruses except ras. J Virol. 1988 Sep;62(9):3540–3543. doi: 10.1128/jvi.62.9.3540-3543.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Sinn E., Pattengale P. K., Wallace R., Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988 Jul 1;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Nakamuta M., Furuich M., Takahashi K., Suzuki N., Endo H., Yamamoto M. Isolation and characterization of a family of rat endogenous retroviral sequences. Virus Genes. 1989 Sep;3(1):69–83. doi: 10.1007/BF00301988. [DOI] [PubMed] [Google Scholar]
- Owen R. D., Ostrowski M. C. Rapid and selective alterations in the expression of cellular genes accompany conditional transcription of Ha-v-ras in NIH 3T3 cells. Mol Cell Biol. 1987 Jul;7(7):2512–2520. doi: 10.1128/mcb.7.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Rasheed S., Norman G. L., Heidecker G. Nucleotide sequence of the Rasheed rat sarcoma virus oncogene: new mutations. Science. 1983 Jul 8;221(4606):155–157. doi: 10.1126/science.6344220. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Lipman D., Andersen P. R., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of the BALB/c murine sarcoma virus transforming gene. J Virol. 1985 Mar;53(3):984–987. doi: 10.1128/jvi.53.3.984-987.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. Promoter and enhancer activities of long terminal repeats associated with cellular retrovirus-like (VL30) elements. Nucleic Acids Res. 1986 Jan 24;14(2):645–658. doi: 10.1093/nar/14.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Saragosti S., Botchan M. Isolation of cellular genes differentially expressed in mouse NIH 3T3 cells and a simian virus 40-transformed derivative: growth-specific expression of VL30 genes. Mol Cell Biol. 1985 Oct;5(10):2590–2598. doi: 10.1128/mcb.5.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Vass W. C., Lowy D. R., Tambourin P. E. Harvey murine sarcoma virus: influences of coding and noncoding sequences on cell transformation in vitro and oncogenicity in vivo. J Virol. 1989 Mar;63(3):1384–1392. doi: 10.1128/jvi.63.3.1384-1392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Lowy D. R., Scolnick E. M. Mapping of transforming region of the Harvey murine sarcoma virus genome by using insertion-deletion mutants constructed in vitro. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4674–4678. doi: 10.1073/pnas.77.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Gonda M. A., De Feo D., Ellis R. W., Nagashima K., Scolnick E. M. Heteroduplex analysis of cloned rat endogenous replication-defective (30 S) retrovirus and Harvey murine sarcoma virus. Virology. 1980 Nov;107(1):89–99. doi: 10.1016/0042-6822(80)90275-5. [DOI] [PubMed] [Google Scholar]



