Abstract
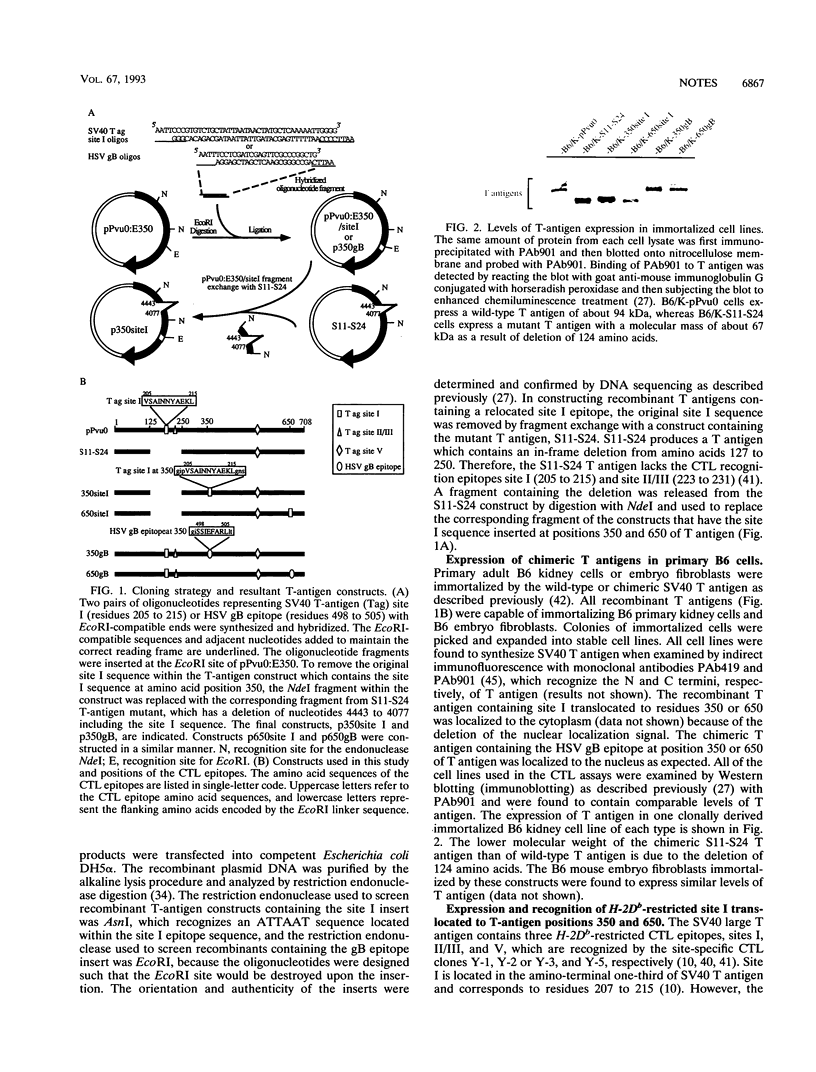
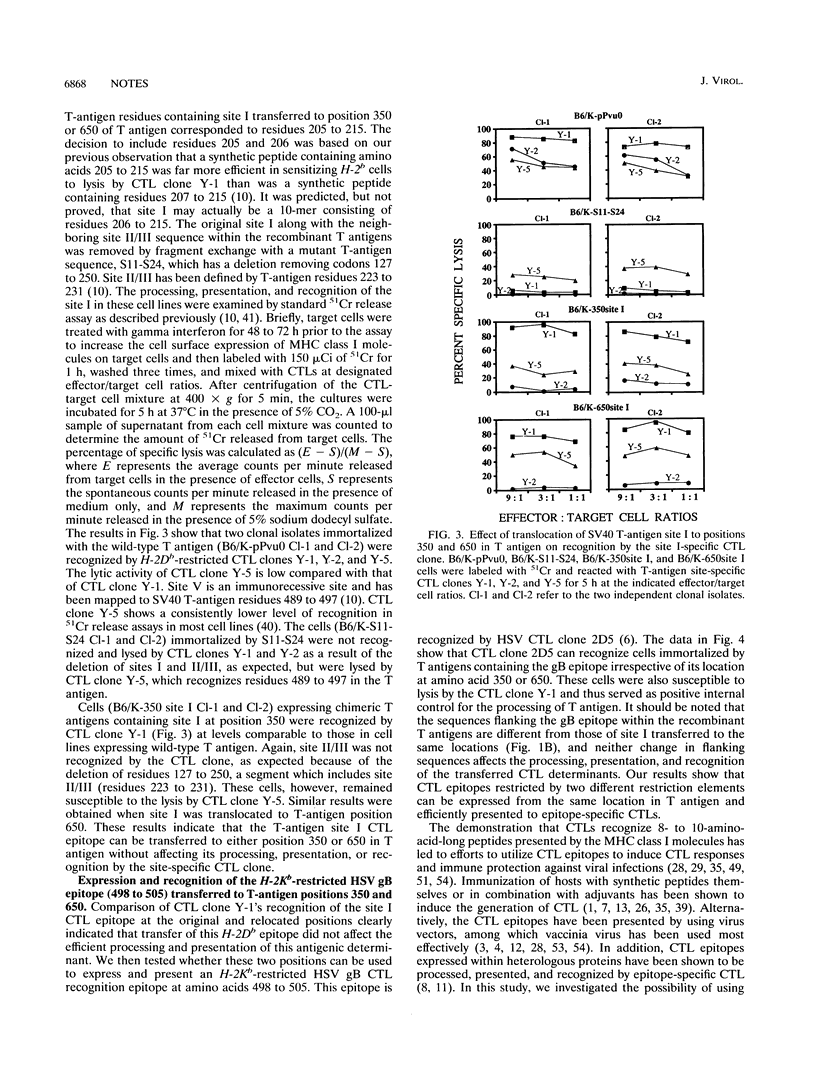
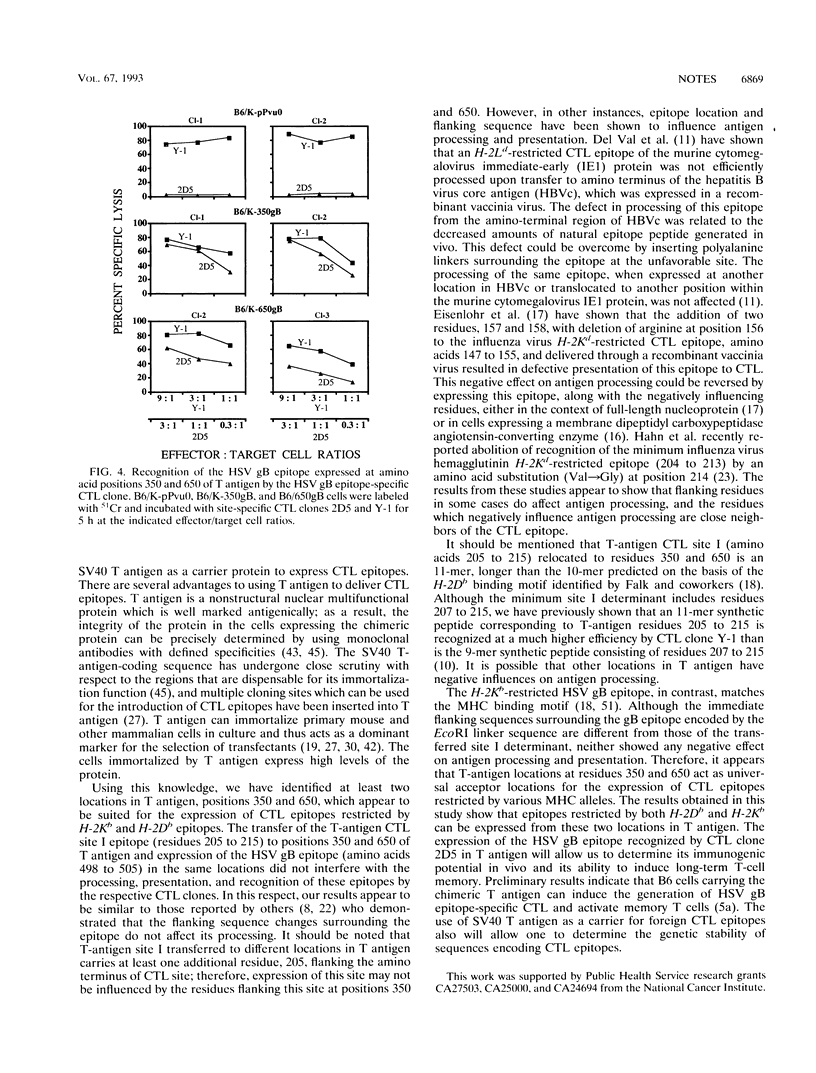
Simian virus 40 (SV40) large T antigen can immortalize a wide variety of mammalian cells in culture. We have taken advantage of this property of T antigen to use it as a carrier for the expression of cytotoxic T-lymphocyte (CTL) recognition epitopes. DNA sequences corresponding to an H-2Db-restricted SV40 T-antigen site I (amino acids 205 to 215) were translocated into SV40 T-antigen DNA at codon positions 350 and 650 containing EcoRI linkers. An H-2Kb-restricted herpes simplex virus glycoprotein B epitope (amino acids 498 to 505) was also expressed in SV40 T antigen at positions 350 and 650. Primary C57BL/6 mouse kidney cells were immortalized by transfection with the recombinant and wild-type T-antigen DNA. Clonal isolates of cells expressing chimeric T antigens were shown to be specifically susceptible to lysis by CTL clones directed to SV40 T-antigen site I and herpes simplex virus glycoprotein B epitopes, indicating that CTL epitopes restricted by two different elements can be processed, presented, and recognized by the epitope-specific CTL clones. Our results suggest that SV40 T antigen can be used as a carrier protein to express a wide variety of CTL epitopes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichele P., Hengartner H., Zinkernagel R. M., Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990 May 1;171(5):1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaya M., Jameson S., Martinez C. K., Hermel E., Aldrich C., Forman J., Lindahl K. F., Bevan M. J., Monaco J. J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992 Feb 13;355(6361):647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Salvucci L. A., Johnson D. C., Tevethia S. S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993 Jul;195(1):62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989 Mar 1;169(3):603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimini G., Pala P., Sire J., Jordan B. R., Maryanski J. L. Recognition of oligonucleotide-encoded T cell epitopes introduced into a gene unrelated to the original antigen. J Exp Med. 1989 Jan 1;169(1):297–302. doi: 10.1084/jem.169.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Deckhut A. M., Lippolis J. D., Tevethia S. S. Comparative analysis of core amino acid residues of H-2D(b)-restricted cytotoxic T-lymphocyte recognition epitopes in simian virus 40 T antigen. J Virol. 1992 Jan;66(1):440–447. doi: 10.1128/jvi.66.1.440-447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Ruppert T., Reddehase M. J., Koszinowski U. H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991 Sep 20;66(6):1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Volkmer H., Messerle M., Reddehase M. J., Koszinowski U. H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991 Jul;65(7):3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Finley D. A controlled breakdown: antigen processing and the turnover of viral proteins. Cell. 1992 Mar 6;68(5):823–825. doi: 10.1016/0092-8674(92)90024-7. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Bacik I., Bennink J. R., Bernstein K., Yewdell J. W. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992 Dec 11;71(6):963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992 Feb 1;175(2):481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992 Mar;66(3):1289–1293. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Gould K., Cossins J., Bastin J., Brownlee G. G., Townsend A. A 15 amino acid fragment of influenza nucleoprotein synthesized in the cytoplasm is presented to class I-restricted cytotoxic T lymphocytes. J Exp Med. 1989 Sep 1;170(3):1051–1056. doi: 10.1084/jem.170.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Braciale V. L., Braciale T. J. Presentation of viral antigen to class I major histocompatibility complex-restricted cytotoxic T lymphocyte. Recognition of an immunodominant influenza hemagglutinin site by cytotoxic T lymphocyte is independent of the position of the site in the hemagglutinin translation product. J Exp Med. 1991 Sep 1;174(3):733–736. doi: 10.1084/jem.174.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Hahn C. S., Braciale V. L., Braciale T. J., Rice C. M. CD8+ T cell recognition of an endogenously processed epitope is regulated primarily by residues within the epitope. J Exp Med. 1992 Nov 1;176(5):1335–1341. doi: 10.1084/jem.176.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierstead T. D., Tevethia M. J. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J Virol. 1993 Apr;67(4):1817–1829. doi: 10.1128/jvi.67.4.1817-1829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Whitton J. L., Joly E., Oldstone M. B. Vaccination and protection from a lethal viral infection: identification, incorporation, and use of a cytotoxic T lymphocyte glycoprotein epitope. Virology. 1990 Oct;178(2):393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Reddehase M. J., Del Val M. Principles of cytomegalovirus antigen presentation in vitro and in vivo. Semin Immunol. 1992 Apr;4(2):71–79. [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Cerundolo V., Colonna M., Cresswell P., Townsend A., DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992 Feb 13;355(6361):644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- Sweetser M. T., Morrison L. A., Braciale V. L., Braciale T. J. Recognition of pre-processed endogenous antigen by class I but not class II MHC-restricted T cells. Nature. 1989 Nov 9;342(6246):180–182. doi: 10.1038/342180a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Anderson R. W., Maloy W. L., Tevethia S. S. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology. 1989 Jul;171(1):205–213. doi: 10.1016/0042-6822(89)90527-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia M. J., Kalderon D., Smith A. E., Tevethia S. S. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology. 1988 Feb;162(2):427–436. doi: 10.1016/0042-6822(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984 Sep;137(2):414–421. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Greenfield R. S., Flyer D. C., Tevethia M. J. SV40 transplantation antigen: relationship to SV40-specific proteins. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):235–242. doi: 10.1101/sqb.1980.044.01.027. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990 Feb;7(1):83–96. [PubMed] [Google Scholar]
- Thompson D. L., Kalderon D., Smith A. E., Tevethia M. J. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology. 1990 Sep;178(1):15–34. doi: 10.1016/0042-6822(90)90375-2. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992 Dec 24;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Oldstone M. B. Class I MHC can present an endogenous peptide to cytotoxic T lymphocytes. J Exp Med. 1989 Sep 1;170(3):1033–1038. doi: 10.1084/jem.170.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Sheng N., Oldstone M. B., McKee T. A. A "string-of-beads" vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993 Jan;67(1):348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



